Session Information
Date: Monday, October 27, 2025
Title: (1553–1591) Systemic Sclerosis & Related Disorders – Clinical Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic sclerosis (SSc) is characterized by a wide range of clinical manifestations and available interventions still lack overall efficacy. Limited cutaneous SSc (lcSSc) and sine scleroderma constitute more than 60% of all SSc patients. Despite this high prevalence, there is a lack of high quality randomized controlled trials conducted for lcSSc. Acquiring high quality data on the natural history of early lcSSc will help design trials with a higher probability of success. CONQUER is a multicenter US-based cohort of patients with early SSc (less than 5 years after first non-Raynaud (RP) symptom), uniquely allowing assessment of the early phases of SSc. This analysis of CONQUER aimed to describe the prevalence of organ involvement in lcSSc patients and to define the onset of key scleroderma manifestations during follow-up. Baseline characteristics predicting disease progression were also identified.
Methods: All patients from the CONQUER multicenter US cohort with physician diagnosed sine scleroderma or lcSSc at baseline were included. Patients were defined as progressors if they experienced one or more of the following events: New renal crisis, Interstitial Lung disease (ILD) progression (relative loss of ≥15% of FVC as compared to first available FVC or onset of FVC less than 80% with ILD), onset of heart failure (LVEF≤40%), pulmonary arterial hypertension on right heart catheterization, gastrointestinal dysmotility requiring enteral or parenteral nutrition, digital ulcer (DU) or gangrene, or death.
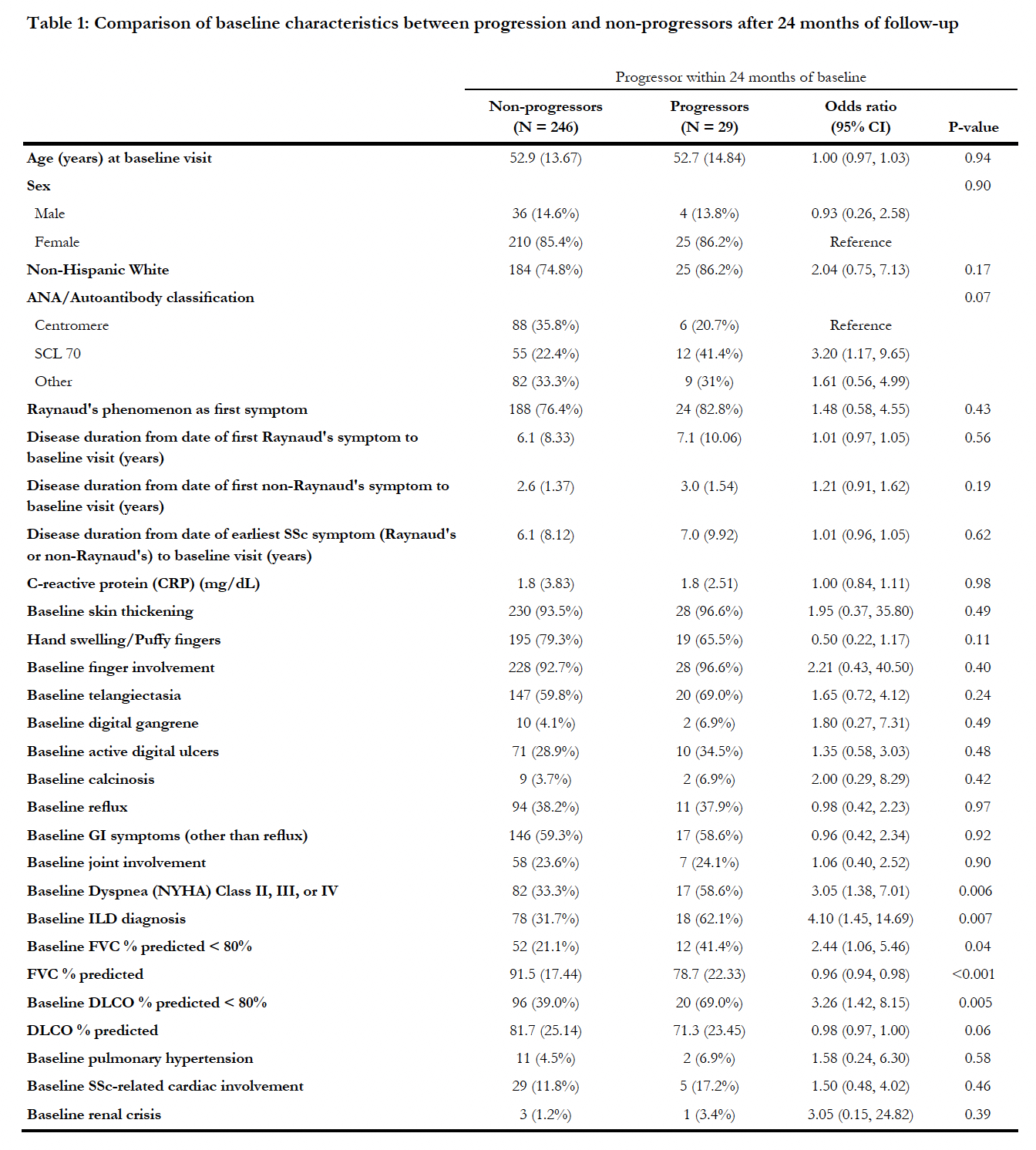
Results: 275 patients were included, with a mean (SD) disease duration of 2.6 (1.39) years since first non-RP and 6.2 (8.51) years since RP. Baseline manifestations included: active digital ulcers in 29.5%, ILD in 34.9%, SSc-related cardiac involvement in 12.4% and renal crisis in 1.5%. Only 14 patients (5%) were identified as progressors in the first 12 month of follow-up. Twenty-nine patients (11%) were identified as progressors in the first 24 months of follow-up. The main reasons for progression at 24 months were ILD progression (55.2%) and new DU (17.2%) during follow-up. Baseline characteristics predicting progression (Table 1) were positivity for anti-topoisomerase-1 antibody (antiTOPO) compared to anti centromere (OR 3.20, 95%CI(1.17-9.65)), the presence of ILD (OR 4.10, 95%CI(1.45-14.69)), the presence of dyspnea (NYHA II, III or IV) (OR 3.05, 95%CI(1.38-7.01), FVC (%pred) less than 80% (OR 2.44, 95%CI(1.06-5.46)), and DLCO (%pred) less than 80% (OR 3.26, 95%CI(1.42-8.15)).
Conclusion: In the CONQUER database of patients with early SSc, 5% and 11% of patients with lcSSc or sine scleroderma experienced disease progression at 12 and 24 months, respectively. This low rate of progression suggests that clinical trials including lcSSc or sine scleroderma patients with early disease may benefit from a trial duration of 24 months. Predictors of progression within the first 24 months included antiTOPO, ILD and cardiopulmonary-related parameters (DLCO, FVC (pred) or dyspnea). Incorporating lcSSc patients with antiTOPO and presence of ILD may help enrich clinical trials with lcSSc patients more likely to show disease progression.
To cite this abstract in AMA style:
Lescoat A, Steen V, Harding M, VanBuren J, Skaug B, Assassi S, Mayes M, McMahan Z, Bernstein E, Castelino F, Chung L, Evnin L, Frech T, Gordon J, Hant F, Hummers L, Lakin K, Lebiedz-Odrobina D, Luo Y, Makol A, Molitor J, Moore D, Richardson C, Sandorfi N, Shah A, Shah A, Volkmann E, Zahn C, Khanna D. Prevalence of Organ Involvement and Baseline Predictors of Disease Progression in Patients with Limited Cutaneous Systemic Sclerosis: Insights from the CONQUER Database [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/prevalence-of-organ-involvement-and-baseline-predictors-of-disease-progression-in-patients-with-limited-cutaneous-systemic-sclerosis-insights-from-the-conquer-database/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-of-organ-involvement-and-baseline-predictors-of-disease-progression-in-patients-with-limited-cutaneous-systemic-sclerosis-insights-from-the-conquer-database/