Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
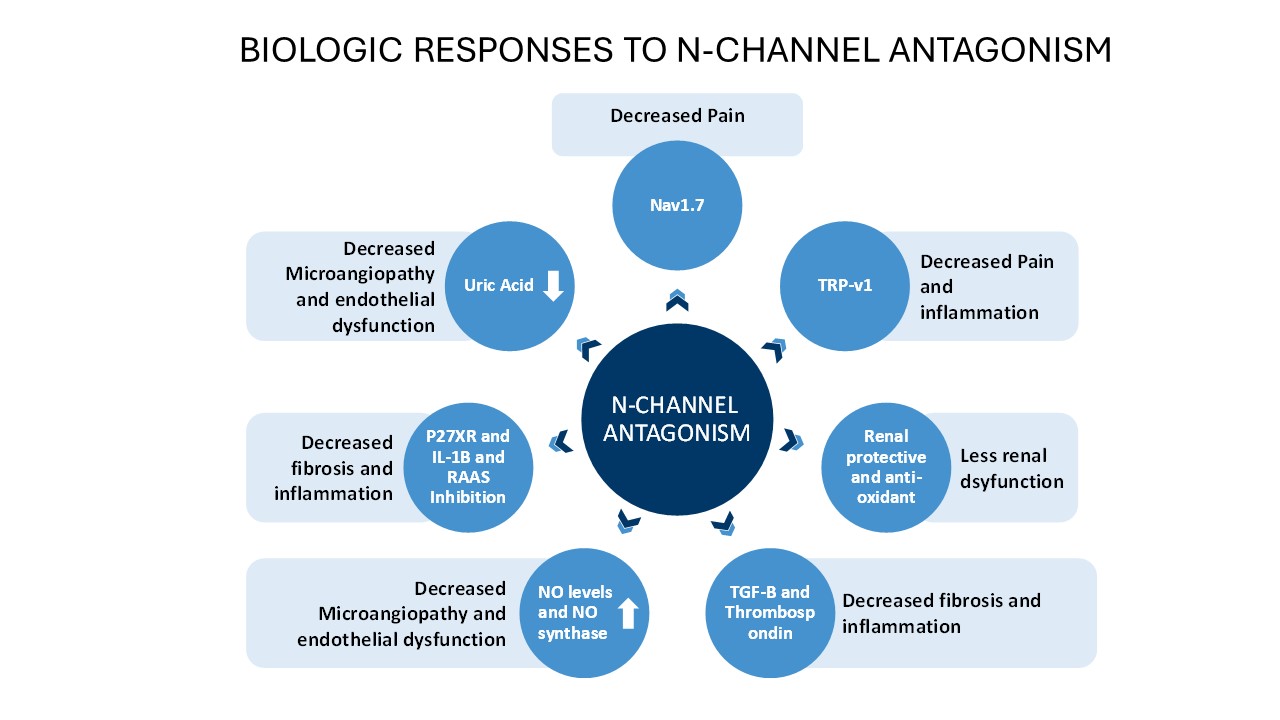
Background/Purpose: While 95% of Systemic Sclerosis (SSc) patients have Raynaud’s phenomenon(RP) and many patients rate it as the most bothersome and severe symptom of their disease, current treatments are only minimally effective and have little disease impact other than a modest reduction in the attack frequency. AISA-021 (cilnidipine) is a novel calcium channel antagonist with significant inhibition of N-type channel activity, conferring a range of downstream biologic effects of possible relevance to treatment of RP and SSc. Here we report preliminary results from a Phase 2A trial in patients with SSc-RP to determine safety and preliminary efficacy of AISA-021 use in patients with SSc-RP. Dose response and the combination with PDE-V inhibition was also evaluated. A comparison with current CCB use for SSc-RP is offered.
Methods: RECONNOITER-1 is a Phase 2, two-part trial, in 76 patients with SSc-RP. The first part of the trial is reported here. Dose groups were AISA-021 10mg and 20mg, alone and with tadalafil 5mg, tadalafil alone and placebo. The primary endpoint was reduction in weekly frequency of RP. Secondary endpoints included Raynaud’s Condition Score (RCS), pain, severity, duration, gastrointestinal symptoms, endothelial function, and the SHAQ. The DSMB met and reviewed data on the first 27 patients.
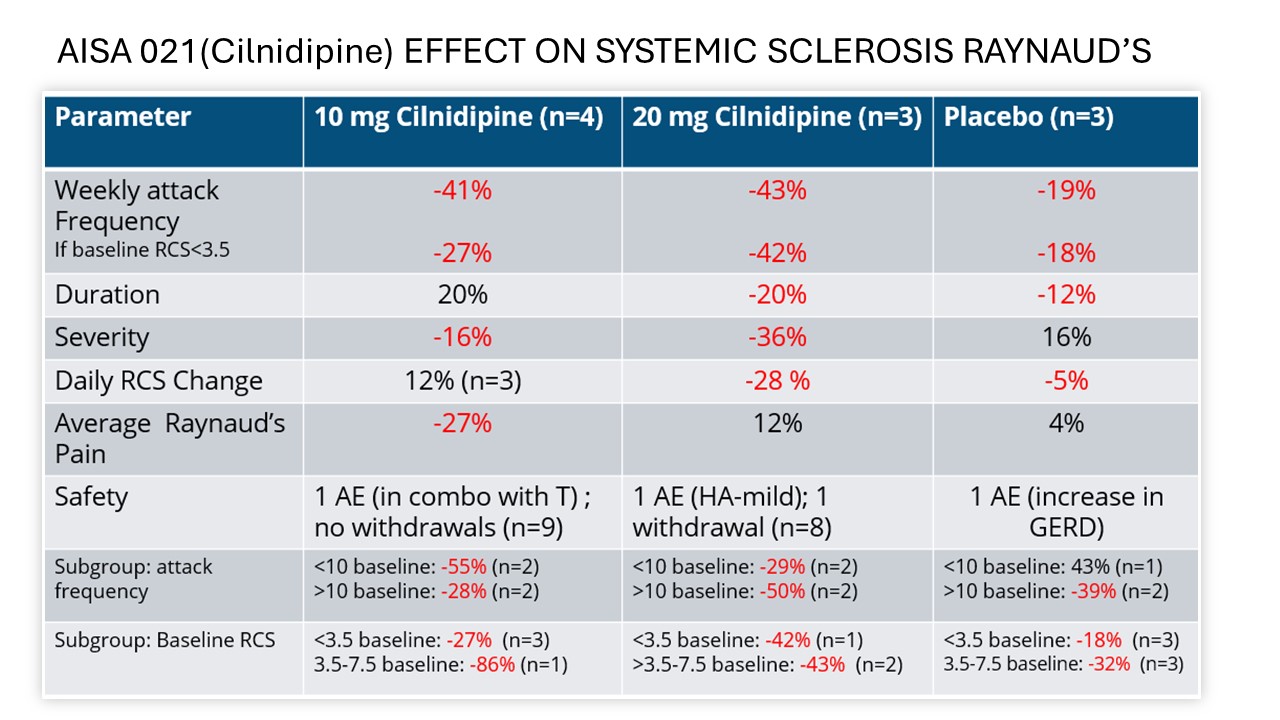
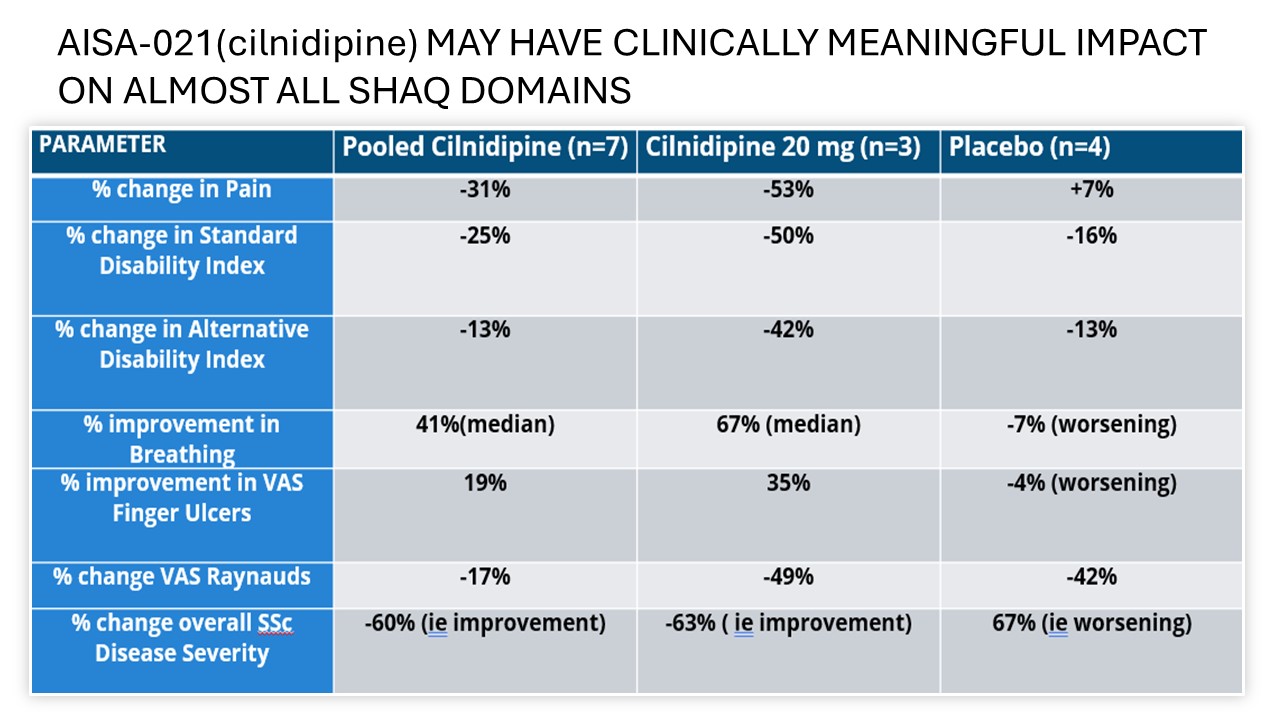
Results: The ITT population included 27 patients; mITT (24) and PP(21) populations were also defined. Results are reported for the mITT population for whom any post treatment data was available. Pooled cilnidipine-only treated patients(n=7) saw a reduction in weekly frequency of attacks of 42.2% versus a reduction of attacks of 18.9% in placebo(n=4) A dose response was seen on the primary endpoint of attack frequency and on severity. Response was also greater in the high dose group on attack duration and RCS. Addition of tadalafil increased treatment effect with 10 mg but not with 20 mg. Cilnidipine at either dose was well tolerated and only Grade 1 mild AEs were reported in 17.6% of cilnidipine-treated patients, with no treatment discontinuations due to adverse events. Results favoring AISA-021 over placebo were seen on all domains of the SHAQ. The DSMB advised terminating Part 1 early based on meeting study goals and Part B will compare Cilnidipine 20mg daily to placebo.
Conclusion: In this preliminary safety and efficacy study, AISA-021 was well-tolerated and efficacy trends were seen. A published metanalysis of CCB use for RP finds AE frequency of >46% and treatment discontinuation in >30% of patients. (P= 0.02 for AISA-021 superiority on safety c/w CCBs). Results replicate the improved 24 year safety record of cilnidipine in hypertension treatment in the SSc-RP population. Increased N-type channel blockade is believed to be responsible for the increased safety with the drug and may produce increased efficacy as well. Additional data will be available from Part 2 of the RECONNOITER study.
To cite this abstract in AMA style:
Sternlicht A, Shanahan M, Morton E, Todd M, Hunt I, Reed Z, weragoda a, Weerakoon L, Briggs E. Preliminary Results from the RECONNOITER Trial, a Phase 2 Study of AISA 021 in the Treatment of Secondary Raynaud’s, Primarily Due to Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/preliminary-results-from-the-reconnoiter-trial-a-phase-2-study-of-aisa-021-in-the-treatment-of-secondary-raynauds-primarily-due-to-systemic-sclerosis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preliminary-results-from-the-reconnoiter-trial-a-phase-2-study-of-aisa-021-in-the-treatment-of-secondary-raynauds-primarily-due-to-systemic-sclerosis/