Session Information
Session Type: Abstract Session
Session Time: 12:00PM-12:15PM
Background/Purpose: Ianalumab, an afucosylated mAb, depletes B cells via enhanced antibody (Ab)-dependent cellular cytotoxicity and blocks B cell-activating factor (BAFF): BAFF-receptor (BAFF-R) mediated signals.1 It is currently being assessed for the treatment of immune-mediated diseases. Given its novel mode of action, it is crucial to assess the effects of ianalumab on preexisting Abs against vaccine antigens (Ags). We evaluated the impact of ianalumab treatment vs placebo on pre-existing Ab levels against 7 pathogens in patients with SLE and Sjögren’s disease (SjD).
Methods: A retrospective analysis was conducted on serum samples from 2 randomized, double-blind (DB), placebo-controlled, phase 2 studies in patients with SjD (NCT02962895) or SLE (NCT03656562). Patients received either placebo or ianalumab 300 mg subcutaneously monthly for 24 weeks (79 with SjD) or for 28 weeks (40 with SLE). In SjD study, patients on 300 mg ianalumab at Week (W) 24 were re-randomized to receive DB monthly ianalumab 300 mg or placebo until W52. Patients on placebo at W24 were switched to a lower ianalumab dose and were excluded from further analysis. In the SLE study, all the patients switched from DB to open label ianalumab up to W52. Abs (IgG isotypes) to vaccine Ags and auto-Abs were measured at baseline (BL), W24 (SjD) or W28 (SLE), and W52. Changes in Ab levels from BL and proportions of patients maintaining protective levels at W52 were assessed. Three patients (2 SjD and 1 SLE) received booster doses against diphtheria and tetanus toxoid (TTd) under ianalumab treatment.
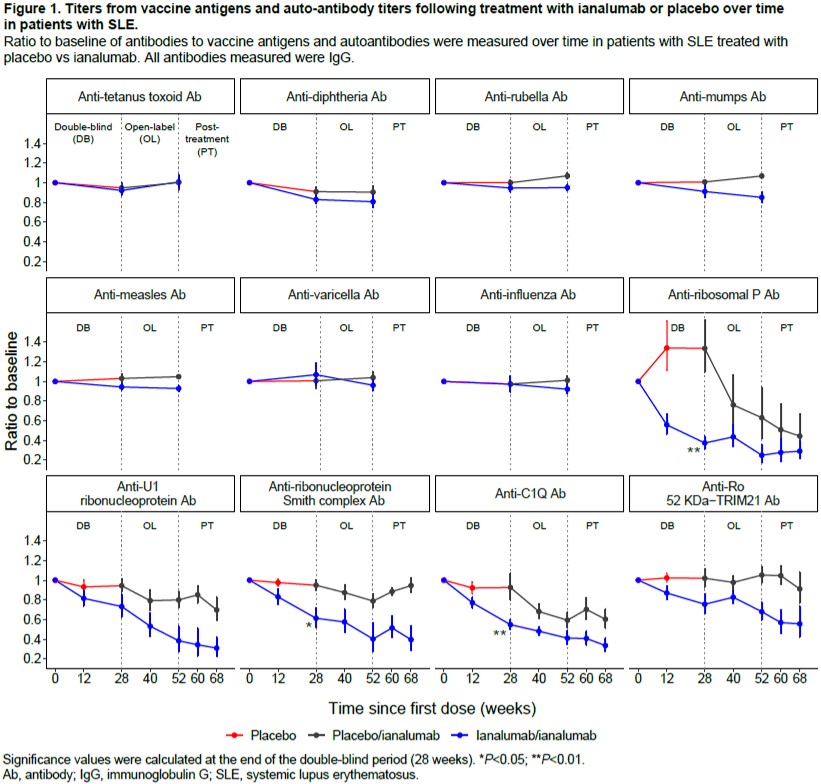
Results: The proportion of patients with SjD and SLE maintaining protective Ab levels against TTd, measles, mumps, varicella, rubella, diphtheria, and influenza remained stable after 52 weeks of ianalumab treatment. In SjD, changes from BL to W24 were < 6% for all Ags in both groups. In SLE, changes from BL to W28 were < 10% in both groups for all Ags besides diphtheria (Fig. 1). For diphtheria, up to 18% changes were seen with ianalumab treatment, likely due to the low preexisting protection (< 50% of patients had protective levels at BL). By contrast, several auto-Abs showed a significant reduction in ianalumab-treated patients (e.g. up to 60% reduction of anti-ribosomal P Abs at W28) compared with placebo (Fig. 1). These results align with ianalumab’s ability to deplete memory and Ab-producing cells,2 while likely not affecting the long-lived bone marrow plasma cells that do not express BAFF-R.3 Among the 3 patients who received booster doses against diphtheria and TTd during ianalumab treatment, 2 showed a subsequent increase in corresponding titers, whereas the other patient received the booster only 10 days before W52 sampling, which explains no increase in titers.
Conclusion: Treatment with ianalumab up to 52 weeks did not result in a reduction of the Ab titers to previous immunizations against tetanus, varicella, measles, mumps, rubella, diphtheria, and influenza while having a clear impact on auto-Ab levels. References McWilliams EM, et al. Blood Adv 2019;3(3):447-460. Dörner T, et al. Ann Rheum Dis 2024;83:956-957. Darce JR, et al. J Immunol 2007;179 (11):7276-7286.
To cite this abstract in AMA style:
Dörner T, Shen N, Grader-Beck T, Walter C, Wioland C, Rauld C, Schmutz P, Riek S, Hueber W, SIPS C, Oliver S, Lau C, Bonal C, Isnardi I. Preexisting Antibodies Against Vaccine Antigens Are Preserved In Patients With Systemic Lupus Erythematosus and Sjögren’s Disease Upon Ianalumab Treatment While Autoantibodies Decline [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/preexisting-antibodies-against-vaccine-antigens-are-preserved-in-patients-with-systemic-lupus-erythematosus-and-sjogrens-disease-upon-ianalumab-treatment-while-autoantibodies-decline/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preexisting-antibodies-against-vaccine-antigens-are-preserved-in-patients-with-systemic-lupus-erythematosus-and-sjogrens-disease-upon-ianalumab-treatment-while-autoantibodies-decline/