Session Information
Date: Saturday, November 16, 2024
Title: B Cell Biology & Targets in Autoimmune & Inflammatory Disease Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Autologous anti-CD19 chimeric antigen receptor (CAR) T cells show early clinical evidence of safety and efficacy for treating several autoimmune diseases (Müller F. N Engl J Med. 2024). Allogeneic CAR T cells bring the promise of an off-the-shelf therapy with the scalability to address the growing B-cell driven autoimmune disease population, no need for patient apheresis, and reduced treatment wait times (Van Blarcom T. CAR TCR Summit. 2023). KYV-201 is an allogeneic anti-CD19 CAR T-cell therapy for B-cell driven autoimmune diseases. KYV-201 combines the same fully human anti-CD19 CAR construct as KYV-101 (a first-in-class autologous CAR T-cell therapy being investigated in patients with autoimmune disease) with a differentiated allogeneic platform developed by Intellia Therapeutics involving selected CRISPR/Cas9-mediated gene edits. These edits intend to avoid graft versus host disease by removing the endogenous T-cell receptor and provide immune evasion to prolong CAR T-cell persistence by removing key cell surface proteins involved in host recognition of an allogeneic product. This study aims to generate clinical-scale drug product characterization and preclinical data to support advancement of KYV-201 to first-in-human clinical studies.
Methods: CAR-mediated and target-dependent activity of KYV-201 against CD19+ target cell lines and primary B cells was assessed with in vitro cytotoxicity, cytokine production, and proliferation assays, and an in vivo xenograft model. Immune evasion editing functionality was assessed by in vitro assays modeling host T and NK cell-mediated rejection mechanisms. Genotoxicity concerns of the product were assessed with off-target editing, chromosomal translocation, karyotyping, and cytokine independent growth assays.
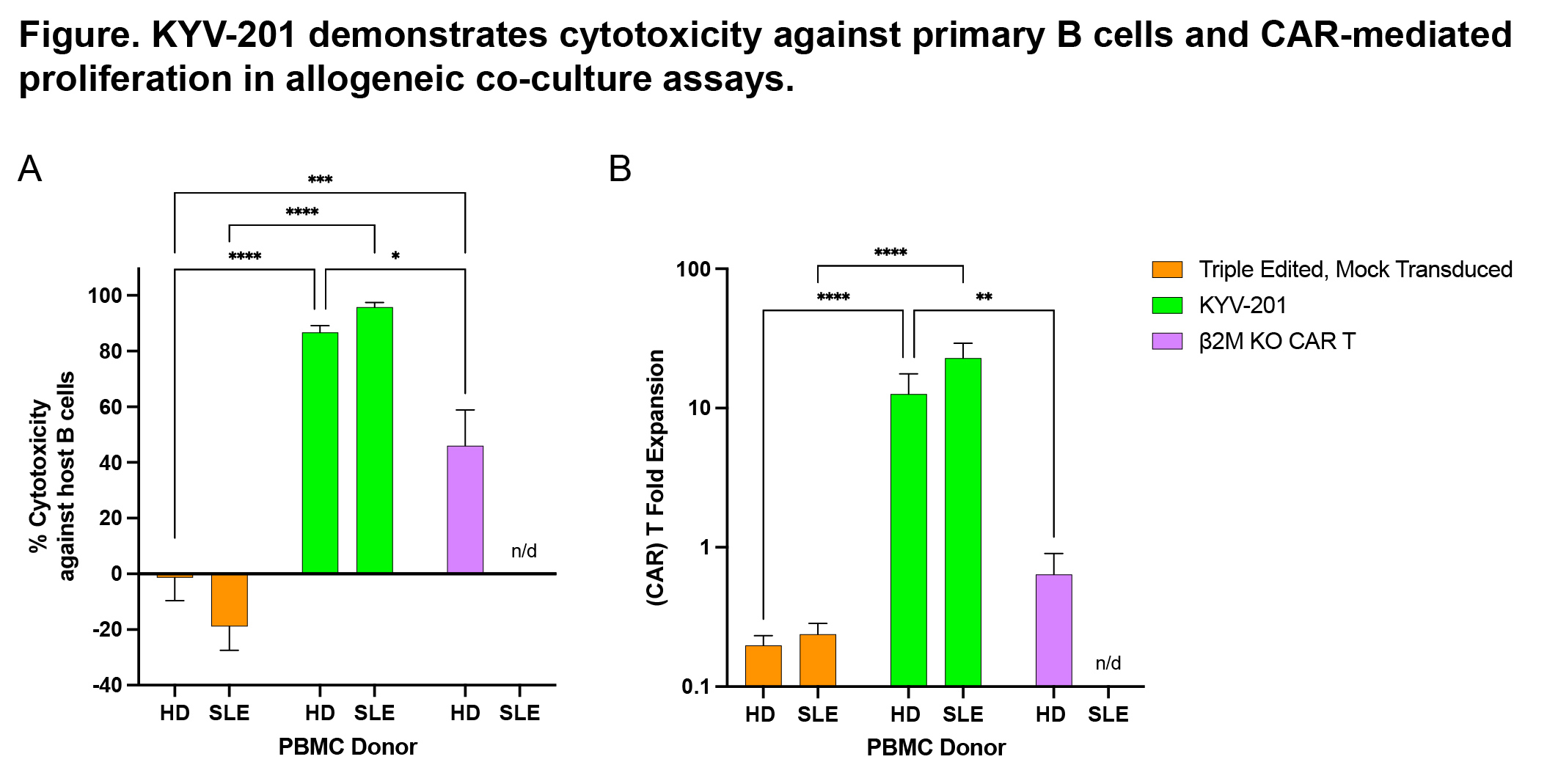
Results: High efficiency CAR transduction and on-target gene editing was achieved in clinical-scale lots of KYV-201 produced under good manufacturing practices. Preclinically, KYV-201 demonstrated CAR-mediated and CD19-dependent cytotoxicity, cytokine production, and proliferation against human CD19+ NALM6 cells. In vivo, KYV-201 controlled CD19+ NALM6 xenograft growth in a dose-dependent manner, similar to unedited anti-CD19 CAR T cells not modified with CRISPR/Cas9-mediated gene edits. In co-culture with allogeneic peripheral blood mononuclear cells from either healthy donors or patients with systemic lupus erythematosus, KYV-201 eliminated B cells and proliferated in a CAR-dependent manner (Figure) without evidence of rejection by alloreactive T and NK cells. Genotoxicity assays performed on clinical-scale lots revealed minimal levels of off-target editing, translocations, or chromosomal abnormalities in KYV-201 and lack of transformation by cytokine-independent growth assays.
Conclusion: The preclinical and drug product characterization data generated for KYV-201 demonstrate the functionality and safety necessary to advance to first-in-human studies, bringing the promise of allogeneic anti-CD19 CAR T cells for autoimmune diseases closer to fruition. A phase 1, open-label, multicenter study of the safety, tolerability, and clinical activity of KYV-201 in refractory lupus nephritis is planned.
Abbreviations: β2M, beta_2-microglobulin; CAR, chimeric antigen receptor; HD, healthy donor; KO, knockout; n/d, no data; PBMC, peripheral blood mononuclear cell; SLE, systemic lupus erythematosus.
To cite this abstract in AMA style:
Mahne A, Rodriguez R, Wang J, Anaya D, Cheng J, Kwong B, Banuelos J, Starokadomskyy P, Park S, Gibson C, Sengupta S, Sandoval S, Bravo J, Flandez J, Shah S, Goodsell A, Khoshnoodi N, Zeng J, Foos-Russ S, Lorente M, Adrian J, Klasson T, Zhang Y, Seitzer J, Schultes B, Van Blarcom T. Preclinical Development and Manufacturability of KYV-201, an Investigational Allogeneic Anti-CD19 Chimeric Antigen Receptor T Cell for the Treatment of Autoimmune Disease [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/preclinical-development-and-manufacturability-of-kyv-201-an-investigational-allogeneic-anti-cd19-chimeric-antigen-receptor-t-cell-for-the-treatment-of-autoimmune-disease/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preclinical-development-and-manufacturability-of-kyv-201-an-investigational-allogeneic-anti-cd19-chimeric-antigen-receptor-t-cell-for-the-treatment-of-autoimmune-disease/