Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Cellular interactions between alveolar macrophages (MΦ) and human lung fibroblasts (HLFs) contribute to excessive pro-inflammatory and pro-fibrotic responses in rheumatoid arthritis interstitial lung disease (RA-ILD), leading to pulmonary fibrosis. Our laboratory has previously shown that malondialdehyde-acetaldehyde (MAA) and citrulline (CIT) modifications co-localize in the lung tissue of RA-ILD patients. However, the mechanism by which MAA and CIT modified proteins alter cellular interactions between MΦ and HLFs has not been well delineated. The purpose of this study was to identify fibrotic gene expression and activation of signaling pathways by HLFs in response to: 1) macrophage supernatants (MΦ-SN) collected post-stimulation with MAA and/or CIT modified fibrinogen (FIB); or, 2) direct stimulation with MAA and/or CIT modified FIB.
Methods: Primary HLFs were treated with either MAA and/or CIT modified FIB or with MΦ-SN collected from PMA-activated U-937 cells following stimulation with MAA/CIT modified FIB. RNA was isolated 8-hours post-treatment and evaluated for the expression of 770 genes using the NanoString® Human Fibrosis Panel. The top 30 genes with the highest values for fold increase (compared to FIB or MΦ-SNFIB) were categorized into four stages (inflammation, initiation, modification, and proliferation) in the Nanostring® software based on their role in the fibrotic pathway, with several genes exerting influence on multiple pathways. Additionally, HLFs were evaluated by Western Blot for phosphorylation of signaling pathways involved in fibrosis.
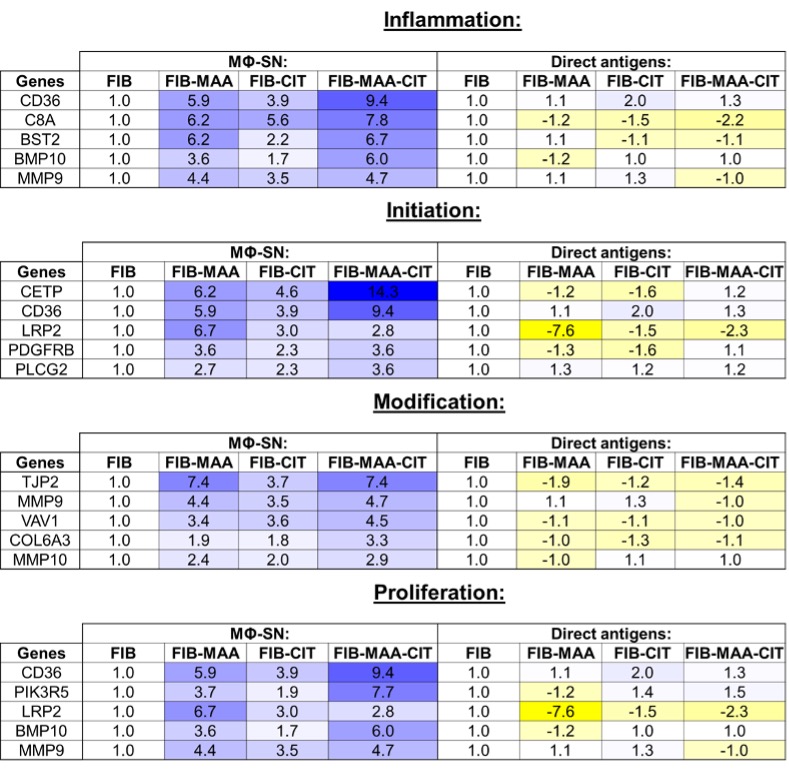
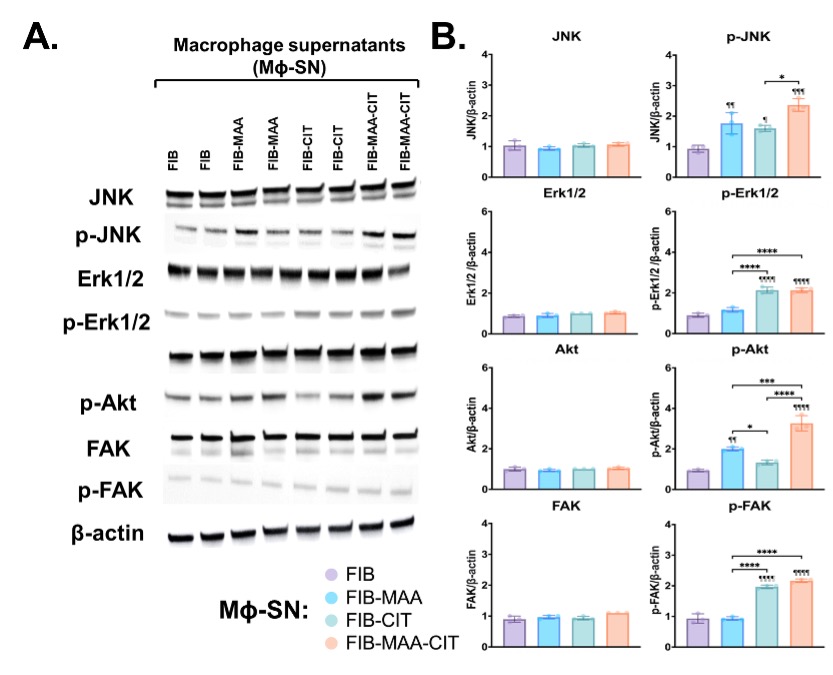
Results: Treatment ofHLFs with MΦ-SN upregulated pro-fibrotic genes in all 4 pathways beyond the effects of direct antigen stimulation (Fig.1). For inflammatory genes, exposure of HLFs to MΦ-SNFIB-MAA-CIT yielded the highest mRNA fold increase vs. other groups. The top 5 inflammatory genes expressed following stimulation with MΦ-SNFIB-MAA-CIT vs. MΦ-SNFIB were CD36 (9-fold; critical in fibroblast activation), C8A (8-fold; component of complement system), BST2 (7-fold; bone marrow stromal antigen), BPM-10 (6-fold; bone morphogenic protein belonging to TGF-b family), MMP9 (5-fold; plays a role in matrix remodeling). The top genes for initiation, modification, and proliferation upregulated in the MΦ-SNFIB-MAA-CIT group were; CETP (14-fold), TJP2 (7-fold), and CD36 (9-fold), respectively. HLFs treated with MΦ-SNFIB-MAA-CIT in comparison to MΦ-SNFIB-MAA and MΦ-SNFIB-CIT showed an increased expression of phosphorylated; (p-) JNK (p < 0.001 vs. MF-SNFIB), p-Erk1/2 (p < 0.0001), p-Akt (p < 0.0001), and p-FAK (p < 0.0001) (Fig.2). Direct antigen stimulation did not upregulate any of the signaling pathways examined (data not shown).
Conclusion: Our studies demonstrate that HLF interaction with MΦ released mediators, particularly those initiated by exposure to dually modified antigens, are necessary to engage all 4 relevant pathways involved in fibroblast-mediated pulmonary fibrosis that characterizes RA-ILD. Identification of MΦ released soluble factors may serve as an important mediator of the inflammatory and fibrotic processes underlying RA-ILD pathogenesis and could serve as a potential novel target for treatment.
To cite this abstract in AMA style:
Aripova N, Duryee M, Hunter C, Butler B, Nelson A, England B, O'Dell J, Poole J, Thiele G, Mikuls T. Post-translationally Modified Fibrinogen Activated Macrophages Drive the Expression of Fibrotic Genes in Human Lung Fibroblasts [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/post-translationally-modified-fibrinogen-activated-macrophages-drive-the-expression-of-fibrotic-genes-in-human-lung-fibroblasts/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/post-translationally-modified-fibrinogen-activated-macrophages-drive-the-expression-of-fibrotic-genes-in-human-lung-fibroblasts/