Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Post-Acute Sequalae of COVID-19 (PASC), prevalent in ~20-30% of convalescent COVID-19 patients is characterized by new/persistent symptoms after the initial recovery. Certain autoantibodies have been described in acute COVID-19 but their clinical significance is unknown. The purpose of this study was the assessment of such antibodies in PASC, their association with symptom persistence as well as the potential for the development of future rheumatic and/or autoimmune diseases.
Methods: In a multi-center, observational study (Post-COVID), we enrolled n=58 PCR+ COVID-19 patients between August’20-September’21 +/- symptoms, at 12 months post-recovery. In a subsequent, observational study (Autoimmunity in Post-Acute COVID Sequelae, AIPACS), between September’21-April’22, we recruited n=58 PASC patients (persistent symptoms for >12 weeks, PCR-confirmed) at 3-6 months (n=11), 6-9 months (n=9), 9-12 months (n=18), and >12 months (n=20) post-infection. All patients were ≥18 years, without history of autoimmune disease. Rapid assessment line immunoassays (HUMAN Diagnostics, Germany) were used to detect circulating autoantibodies.
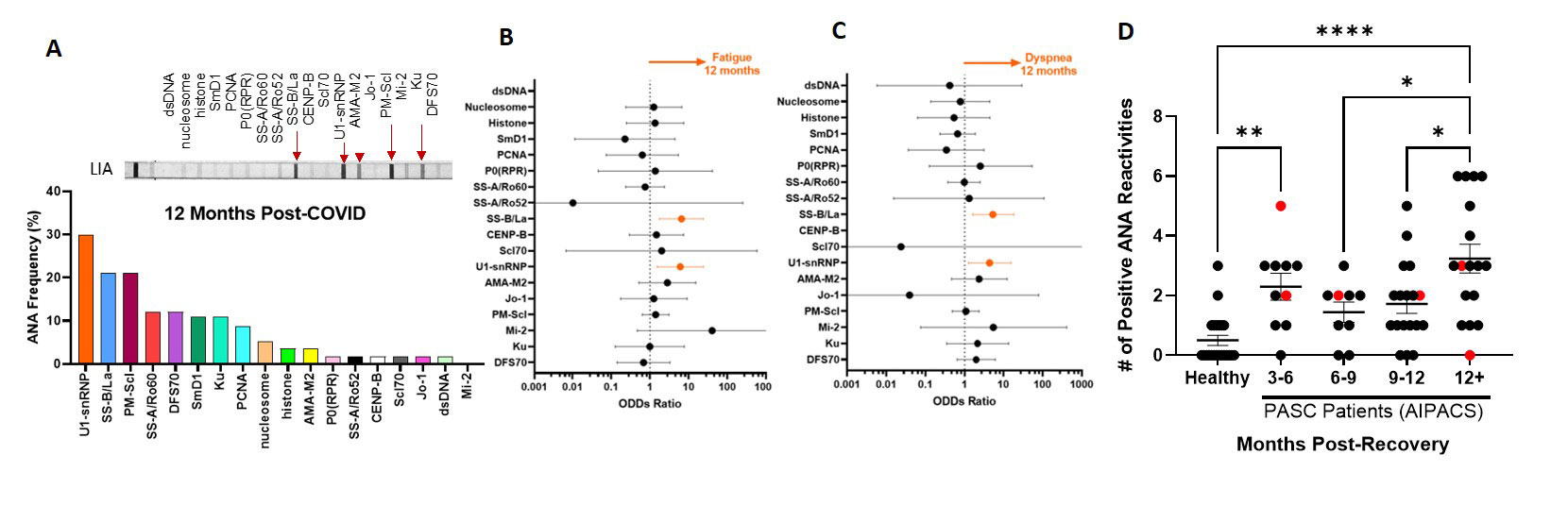
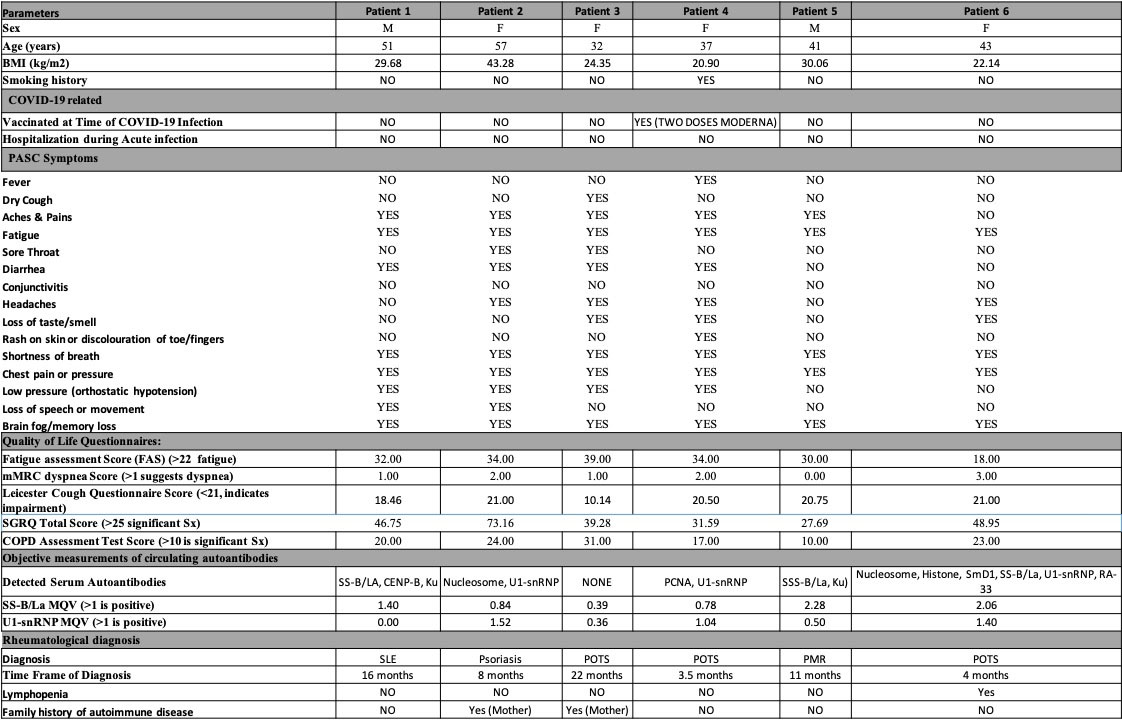
Results: In the Post-COVID study, 43% (25/58) of patients reported symptoms (fatigue, dyspnea, or cough) at 12 months post-infection. Anti-U1-snRNP (30%) and anti-SS-B/La (21%) were the most prevalent autoantibodies (Fig. 1A) that positively correlated with and predicted persisting symptoms of fatigue and dyspnea at 12-month post-infection (simple logistic regression, P< 0.05) (Fig. 1B, C). In the AIPACS study, patients at >12 months post-infection had significantly higher numbers of positive autoreactivities compared to patients 6-12 months post-infection (p=0.03) (Fig. 1D). Finally, six patients (10%, 2 males/4 females, median age 42 years) were newly diagnosed with an autoimmune and/or rheumatic disease; systemic lupus erythematosus (n=1), postural orthostatic tachycardia syndrome (n=3), psoriasis (n=1) and polymyalgia rheumatica (n=1) (Table 1). Anti-SS-B/La and/or anti-U1-snRNP were detectable in 5/6 patients.
Conclusion: Autoantibodies to certain nuclear antigens were detected in PASC patients beyond 12 months post-infection. A subset of PASC patients without prior history of autoimmunity may be susceptible to new-onset rheumatological complications.
To cite this abstract in AMA style:
Thanavala N, Son K, Sedhom N, Somalwar S, Patel Z, Jamil R, Venegas C, Thakar A, Yuen A, Kjarsgaard M, Waserman S, Duong M, Nair P, Carlsten C, Larche M, Ho T, Svenningsen S, TSELIOS K, Mukherjee M. Post-Acute COVID-19 Sequalae (PACS) with New-onset Rheumatological Complications [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/post-acute-covid-19-sequalae-pacs-with-new-onset-rheumatological-complications/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/post-acute-covid-19-sequalae-pacs-with-new-onset-rheumatological-complications/