Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Polynesian (NZ Māori and Pacific) populations have increased prevalence of gout. Hyperuricaemia is contributed to by increased urate production in the liver via the purine pathway. PRPSAP1 (phosphoribosyl pyrophosphate (PRPP) synthetase-associated protein 1) binds PRPP synthetase and negatively regulates the production of PRPP and urate. Genetic variants in close proximity to PRPSAP1 have been associated with serum urate and colocalize with an expression quantitative trait locus (eQTL) for PRPSAP1 (Boocock et al. 2020 PMID 31985003), implicating PRPSAP1 as a causal gene for hyperuricemia. We hypothesize that functional variants within genes that function in the purine pathway (PRPS1 and PRPSAP1) could contribute to the risk of gout.
Methods: PRPS1 and PRPSAP1 were sequenced from 811 individuals of Polynesian or European ancestry in order to identify protein-altering variants. A second cohort of 3203 individuals of Polynesian ancestry of gout cases and controls was used to test association with gout using TaqMan®. eQTL data from the latest Genotype Tissue Expression Project release (GTEx v8), that now includes kidney, was used to test for a kidney eQTL.
Results: Two variants within PRPSAP1 predicted to be protein altering and splice site acceptor variants (rs749392722 and rs201995246, respectively) were identified. No protein-altering variants were identified in PRPS1. rs749392722 is a frameshift variant, found only in 19 Polynesian participants, predicted to abolish almost the entirety of exon 3 of PRPSAP1. rs201995246 was identified in one hyperuricaemic male (8.1 mg/dL) of European ancestry. In the larger cohort of Māori and Pacific cases and controls (n = 3203) rs74939722 had a minor allele frequency of 0.025. rs749392722 significantly associates with increased risk of gout in Eastern Polynesian people (OR = 2.074, p = 9.0 x 10-3) and this association was confirmed in a meta-analysis (OR = 2.19, Poverall = 4.0 x 10-3) carried out on all Polynesian groups.
The maximal urate-associated SNP at the PRPSAP1 locus, identified previously by genome-wide association study (rs164009; Boocock et al.), is tightly correlated with the maximally associated SNPs for the PRPSAP1 eQTL in Kidney Cortex (Figure 1). rs164009_A raises serum urate and increases PRPSAP1 expression in the Kidney Cortex. In contrast rs164009 is not amongst the maximally associated SNPs at the PRPSAP1 eQTL in Liver, where there is an eQTL with a different genetic pattern of genetic control.
Conclusion: A PRPSAP1 frameshift Polynesian genetic variant increases risk to gout perhaps by increased production of urate in the liver. The GTEx expression data indicate that the common serum urate association at PRPSAP1 overlaps a kidney eQTL for PRPSAP1, where the urate-raising allele increases PRPSAP1 expression, which would be expected to decrease production of urate via the purine pathway. PRPSAP1 expression in the kidney may be important in serum urate control and this could be independent of any role of PRPSAP1 in regulating PRPP synthetase activity in the liver.
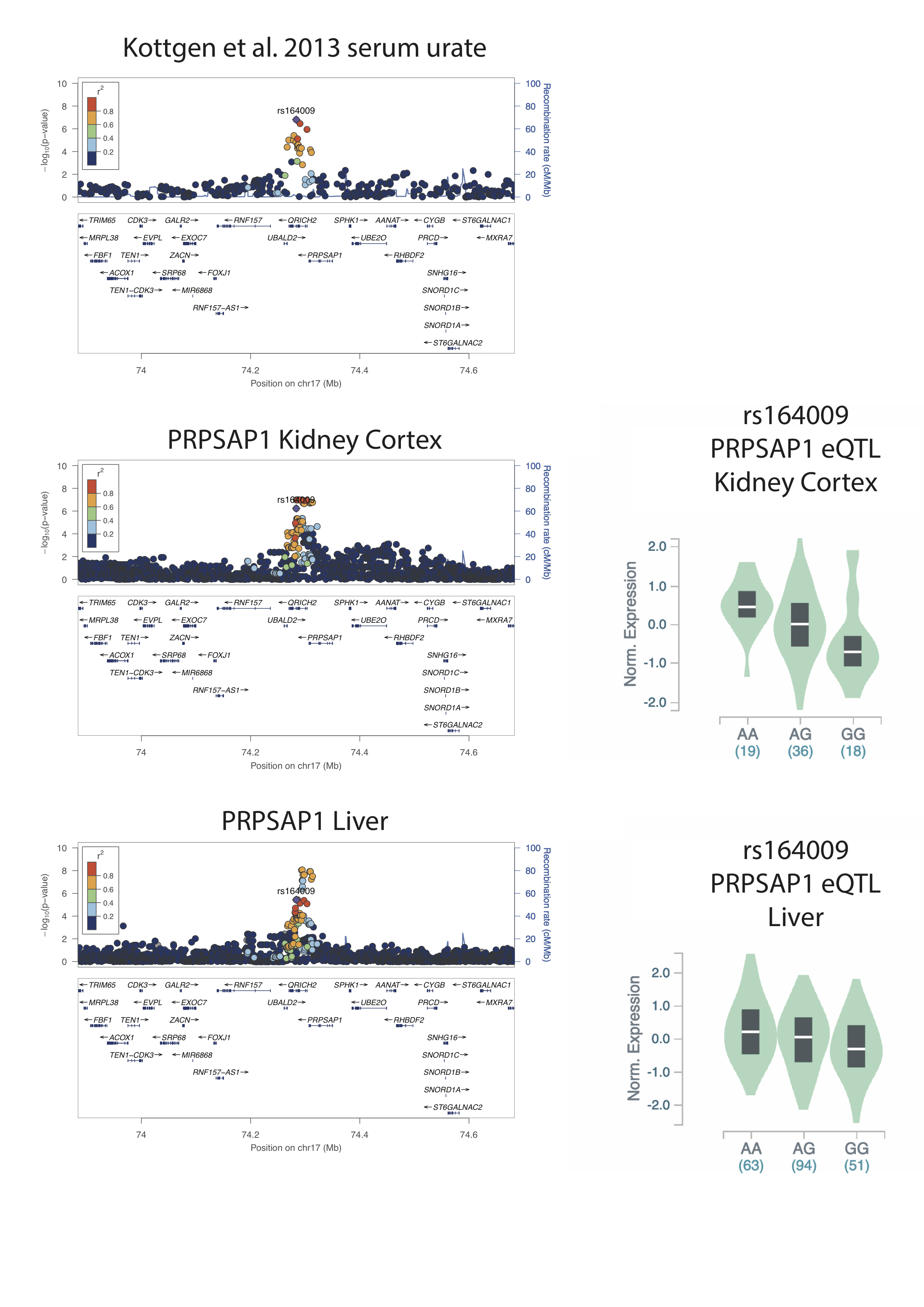 Figure 1. Regional association plots for serum urate (Köttgen et al. 2013) and gene expression of PRPSAP1 in Kidney Cortex and Liver tissue. eQTL violin plots for directionality of expression for the lead Köttgen SNP at PRPSAP1.
Figure 1. Regional association plots for serum urate (Köttgen et al. 2013) and gene expression of PRPSAP1 in Kidney Cortex and Liver tissue. eQTL violin plots for directionality of expression for the lead Köttgen SNP at PRPSAP1.
To cite this abstract in AMA style:
Leask M, Dalbeth N, Stamp L, Merriman T, Phipps-Green A, Topless R, Boocock J, Choi H, Leaupepe K, Stahl E. Polynesian-Specific Gout-Associated Frameshift Variant in PRPSAP1 [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/polynesian-specific-gout-associated-frameshift-variant-in-prpsap1/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/polynesian-specific-gout-associated-frameshift-variant-in-prpsap1/
