Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: In SLE, platelet activation (express P-selectin+) follows the disease activity, which plays a role in thrombosis as well as in inflammation (1). Neutrophils express high levels of PSGL-1, the ligand for P-selectin, which predicts a physical interaction between neutrophils and activated platelets in SLE patients. Our objective is to explore the relationships between platelets and neutrophils in human SLE.
Methods: We prospectively included SLE patients satisfying the 2019 ACR-EULAR criteria. Clinical data including disease activity (SLEDAI-2K; active SLE if SLEDAI ≥6) and immunological parameters were retrieved. We conducted spectral cytometry (Cytek Aurora) on fresh whole blood using a 37-color panel including platelets, neutrophils, and other immune surface markers. Neutrophils were CD66b+ cells identified on the SSC/FSC gating. Platelet-neutrophil aggregates (PNA) were identified as neutrophils coexpressing CD41a ± CD62P (P-selectin) platelet-specific markers. Using FlowJo v10, we conducted supervised and unsupervised analysis of neutrophils based on their interaction with platelets to explore neutrophils heterogeneity. In vitro experiments were conducted to investigate the signaling induced by P-selectin engagement on neutrophils and the impact on their phenotype.
Results: We recruited 50 patients with repeat samples for 8 patients. Platelet-neutrophil aggregates are increased in patients with active SLE compared to inactive SLE and healthy donors (p = 0.005 and p < 0.001, respectively). PNA positively correlate with the SLEDAI (Spearman’s r = 0,49; p = 0.003) and follows disease activity in longitudinal analyses.
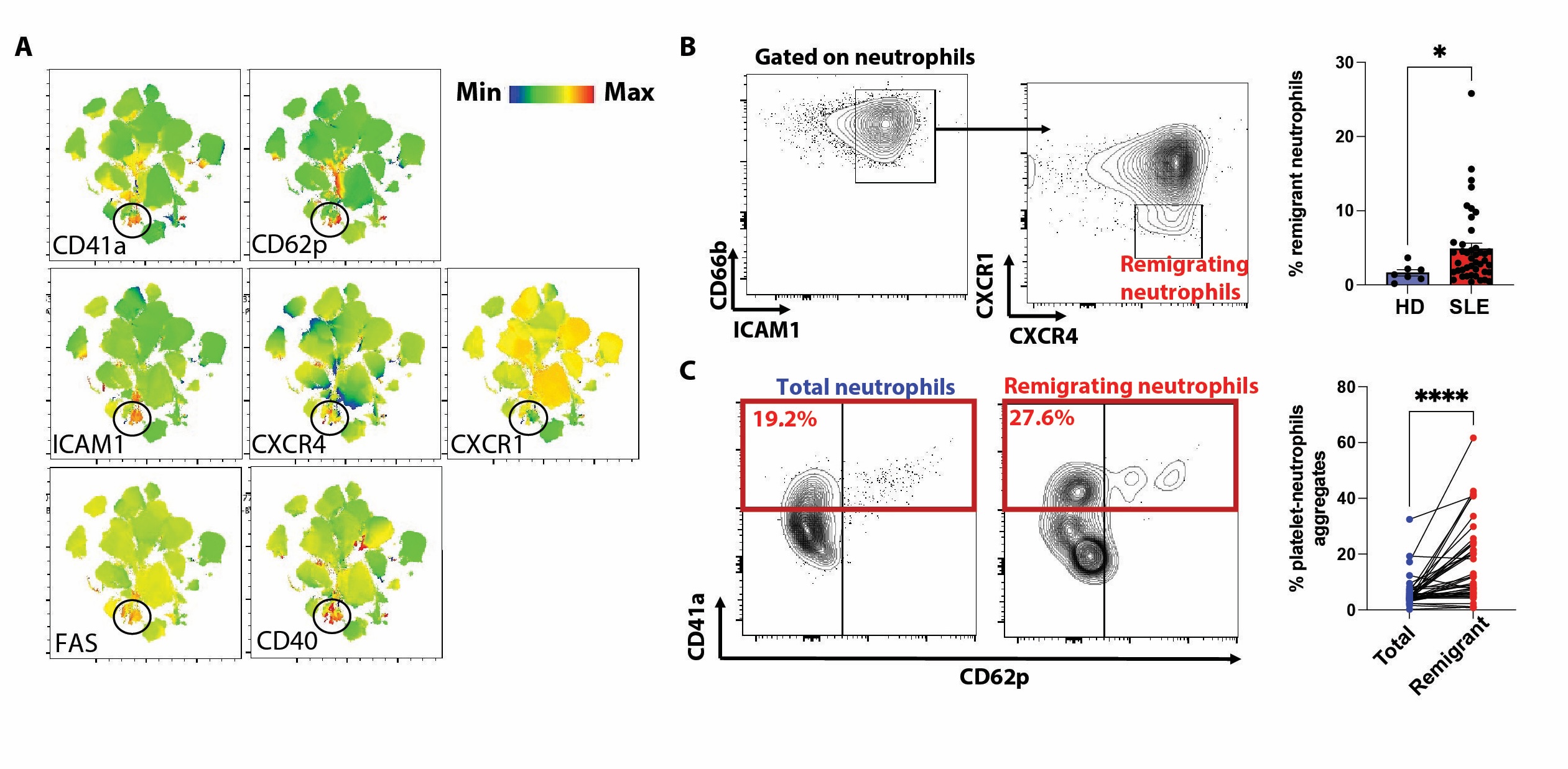
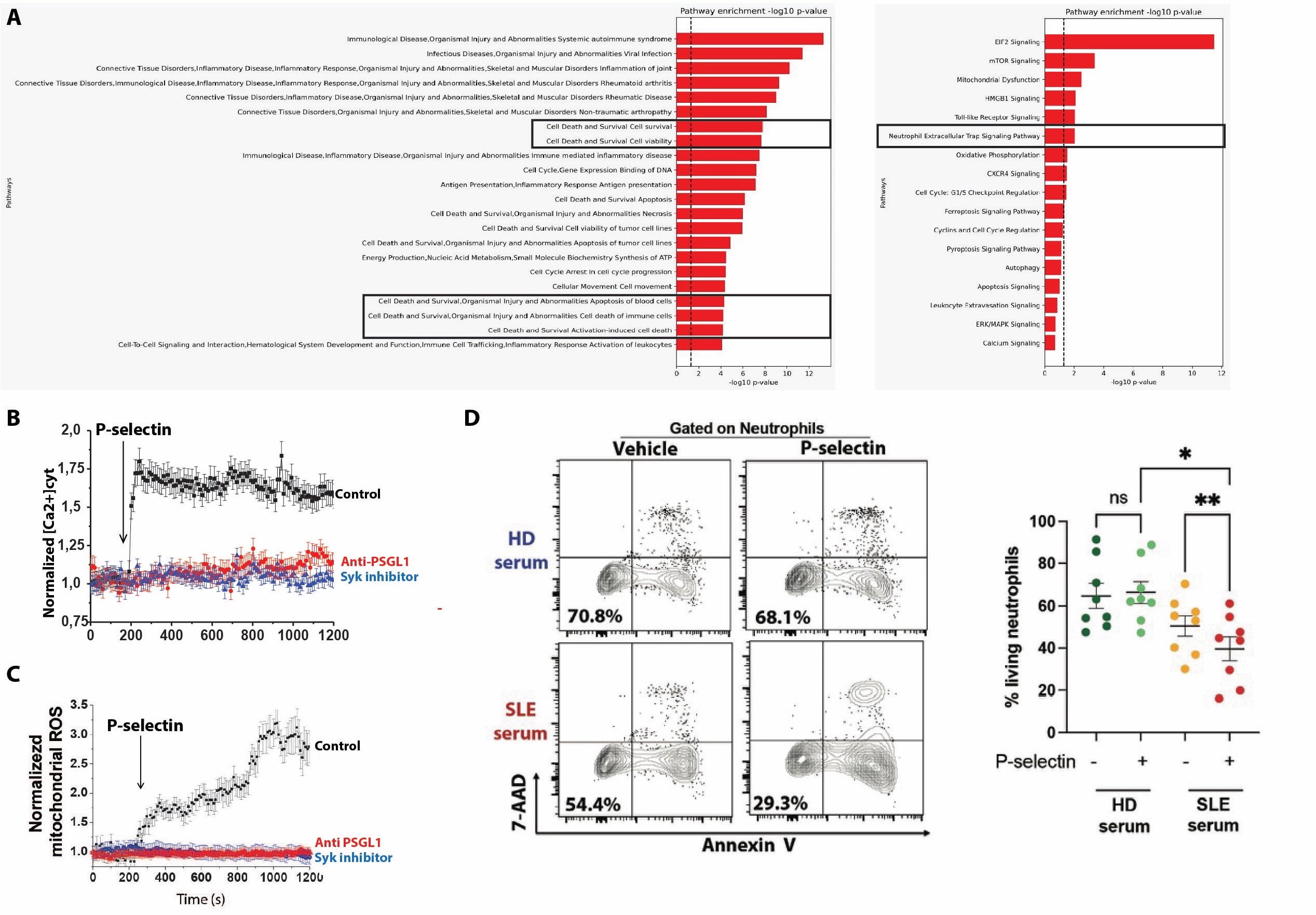
A non supervised analysis identified that platelet-neutrophils aggregates have an atypical phenotype (ICAM1high, CXCR4high, CXCR1low; Fig. 1A), previously identified as activated neutrophils that remigrates from a peripheral tissue (ie, skin) to the kidney to induce tissue inflammation (2). We confirmed that these atypical neutrophils are increased in SLE patients (p < 0.05; Fig. 1B) and that these remigrant neutrophils preferentially aggregate with platelets (p < 0.0001; Fig. 1C). In vitro, P-selectin engagement on human neutrophils induces a PSGL-1/Syk/calcium/mitochondrialROS intracellular signalling which induces the upregulation of genes involved in NETosis (Fig 2A-C). Furthermore, P-selectin engagement promotes SLE-serum induced neutrophil death in vitro (p < 0.01; Fig 2D). Finally, in patients with lupus nephritis, scRNAseq whole-blood study successfully identified PNA and spatial transcriptomics (10X Visium) study of kidney biopsies is ongoing.
Conclusion: Platelet-neutrophil aggregates are increased in SLE and are enriched in an atypical subpopulation of neutrophils believed to remigrate to SLE target organs. Through P-selectin signalling, platelet promotes neutrophil immunogenic death in SLE and represents a novel therapeutic target.
References:
1) Scherlinger et al. Nat Rev Immunol 2023 Aug;23(8):495-510
2) Skopelja-Gardner et al. Proc. Natl. Acad. Sci. 2021 Jan 19;118(3):e2019097118.
(A) UMAP of neutrophils from 20 active SLE patients showing expression patterns of platelet markers (CD41a & CD62p), adhesion marker/chemokine receptors (ICAM1, CXCR4, CXCR1) and receptors involved in neutrophil cell death (CD40, FAS). (B) Gating strategy and cumulative results of ICAM1high CXCR4high CXCR1low (remigrating) neutrophils in SLE patients. (C) Platelet neutrophil aggregates in total and remigrating neutrophils in SLE patients. *, p < 0.05 using Mann-Whitney test; ****, p < 0.0001 using Wilcoxon's test.
To cite this abstract in AMA style:
nansion M, Schmauch E, Vacher P, Sibilia J, Broussaudier N, Bruchon A, Grenot P, Korganow A, Martin T, Caillard S, Duval A, Gottenberg J, Carapito R, Bahram S, Blanco P, Scherlinger M. Platelets Specifically Interact with Remigrating Neutrophils and Promote Immunogenic Cellular Death in Systemic Lupus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/platelets-specifically-interact-with-remigrating-neutrophils-and-promote-immunogenic-cellular-death-in-systemic-lupus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/platelets-specifically-interact-with-remigrating-neutrophils-and-promote-immunogenic-cellular-death-in-systemic-lupus/