Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: The level of placebo group response in clinical trials for chronic pain conditions is a concern for the development of novel analgesics1. Here, we investigate the placebo group responses over time in data from the clinical studies of tanezumab, a monoclonal antibody against nerve growth factor which is intended for the relief of signs and symptoms of moderate to severe osteoarthritis (OA) in adult patients for whom use of other analgesics is ineffective or not appropriate.
Methods: Tanezumab studies from 2008 to 2018 were included in these analyses. Those completed prior to 2015 utilized intravenous (IV) administration, while those conducted after 2015 used subcutaneous (SC) administration. We used data from IV and SC studies to create two cohorts to examine the change in placebo group responses over time. Data from 4 studies (NCT00733902, NCT00744471, NCT00830063 and NCT00863304), conducted between 2008–2010, were pooled to create the IV cohort (n=1814). The SC cohort (n=1545) comprised data from 2 studies, conducted between 2016–2018 (NCT02697773 and NCT02709486). Although there were some differences in the patient populations in the IV and SC cohorts, all studies enrolled patients with a diagnosis of OA in the hip or knee, baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain subscale scores of ≥5 in the index joint and Patient Global Assessment of OA (PGA-OA) of “fair”, “poor” or “very poor”. A WOMAC Physical Function score ≥5 at baseline was also required, with the exception of IV studies NCT00830063 and NCT00863304, which required a score ≥4. Treatments were administered every 8 weeks for 16 ([IV: NCT00830063 and NCT00863304] [SC: NCT02697773]) or 24 weeks [IV: NCT00733902 and NCT00744471] [SC: NCT02709486]).
Results: The percentage of patients who discontinued from the SC cohort for any reason and particularly for insufficient clinical response was lower, compared with the IV cohort (Table 1). The change from baseline to week 16 in the efficacy endpoints of WOMAC Pain, WOMAC Physical Function, PGA-OA, weekly average pain score and WOMAC Stiffness revealed consistently greater placebo group improvements in the SC cohort, compared with the IV cohort (Figure 1).
Conclusion: These data showed an increased placebo group response in patients receiving SC administration in a later time period (2016-2018), compared with IV administration in an earlier time period (2008-2010) across the tanezumab development program. Further analyses will be presented to examine the factors that account for these differences, for example, changes in the route of administration, temporal trends in the patients enrolling in the trials or in the study sites and differences in clinical trial design. These analyses may help advance drug development by identifying critical factors that may increase placebo group responses and thereby limit the assay sensitivity of clinical trials to identify truly efficacious pain treatments.
These studies were sponsored by Pfizer and Lilly. Medical writing support was provided by Steven Moore, PhD, of Engage Scientific Solutions and was funded by Pfizer and Lilly.
References
- Tuttle et al., Pain; 2015; 156:2616-26.
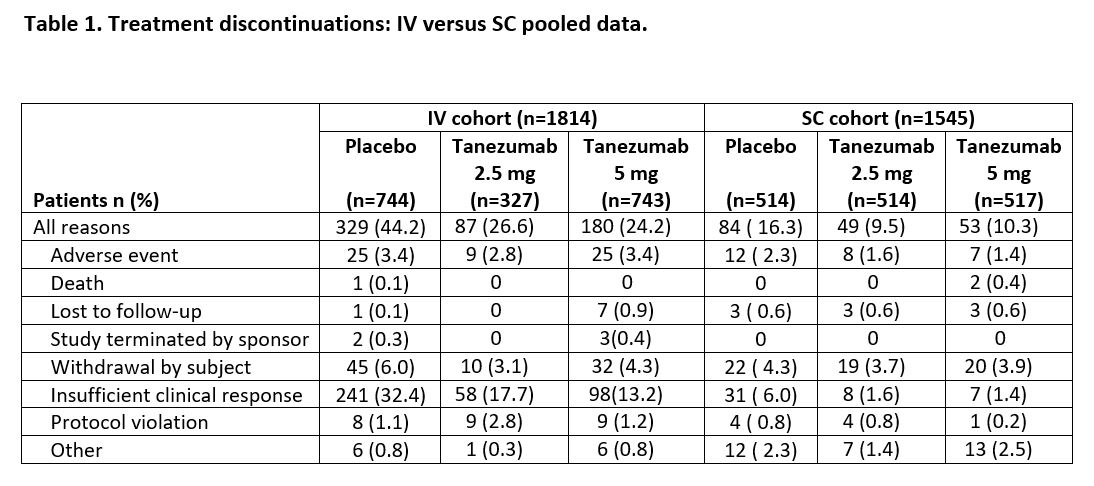 The incidences of withdrawal by reason were determined through completion of the treatment period of each study. IV, intravenous; SC, subcutaneous
The incidences of withdrawal by reason were determined through completion of the treatment period of each study. IV, intravenous; SC, subcutaneous
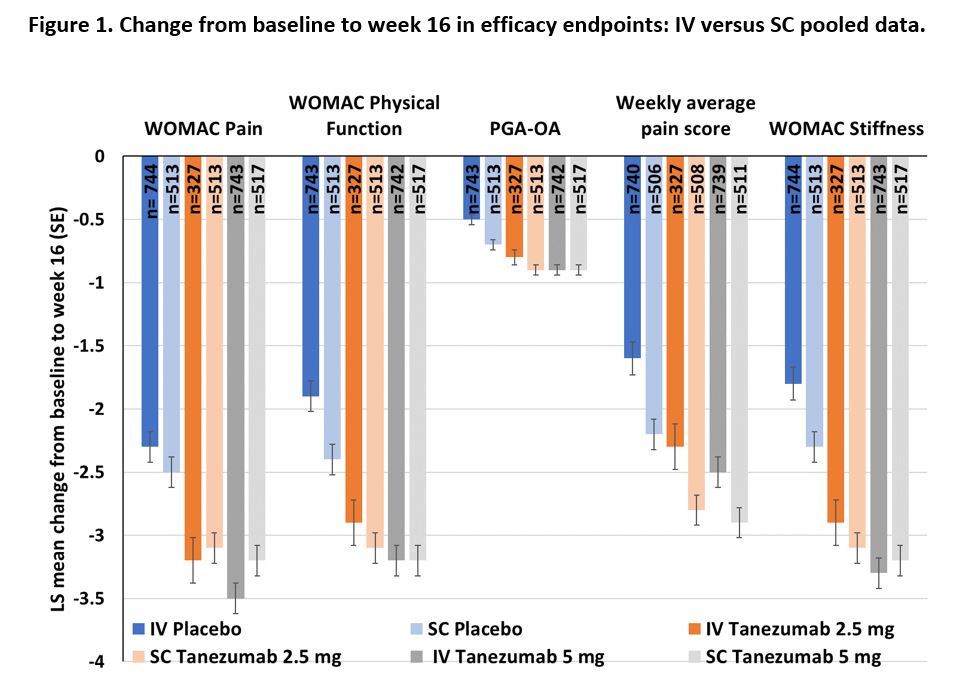 A change from baseline < 0 represents an improvement in all outcomes. IV, intravenous; LS, least squares; PGA-OA, patient global assessment of osteoarthritis; SC, subcutaneous; SE, standard error; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index
A change from baseline < 0 represents an improvement in all outcomes. IV, intravenous; LS, least squares; PGA-OA, patient global assessment of osteoarthritis; SC, subcutaneous; SE, standard error; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index
To cite this abstract in AMA style:
Colloca L, Dworkin R, Farrar J, Tive L, Whalen E, Yang J, Viktrup L, Brown M, West C, Verburg K. Placebo Group Responses in Clinical Trials of Patients with Osteoarthritis: Data from the Tanezumab Development Program [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/placebo-group-responses-in-clinical-trials-of-patients-with-osteoarthritis-data-from-the-tanezumab-development-program/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/placebo-group-responses-in-clinical-trials-of-patients-with-osteoarthritis-data-from-the-tanezumab-development-program/
