Session Information
Date: Sunday, November 12, 2023
Title: (0609–0672) Systemic Sclerosis & Related Disorders – Clinical Poster I: Research
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
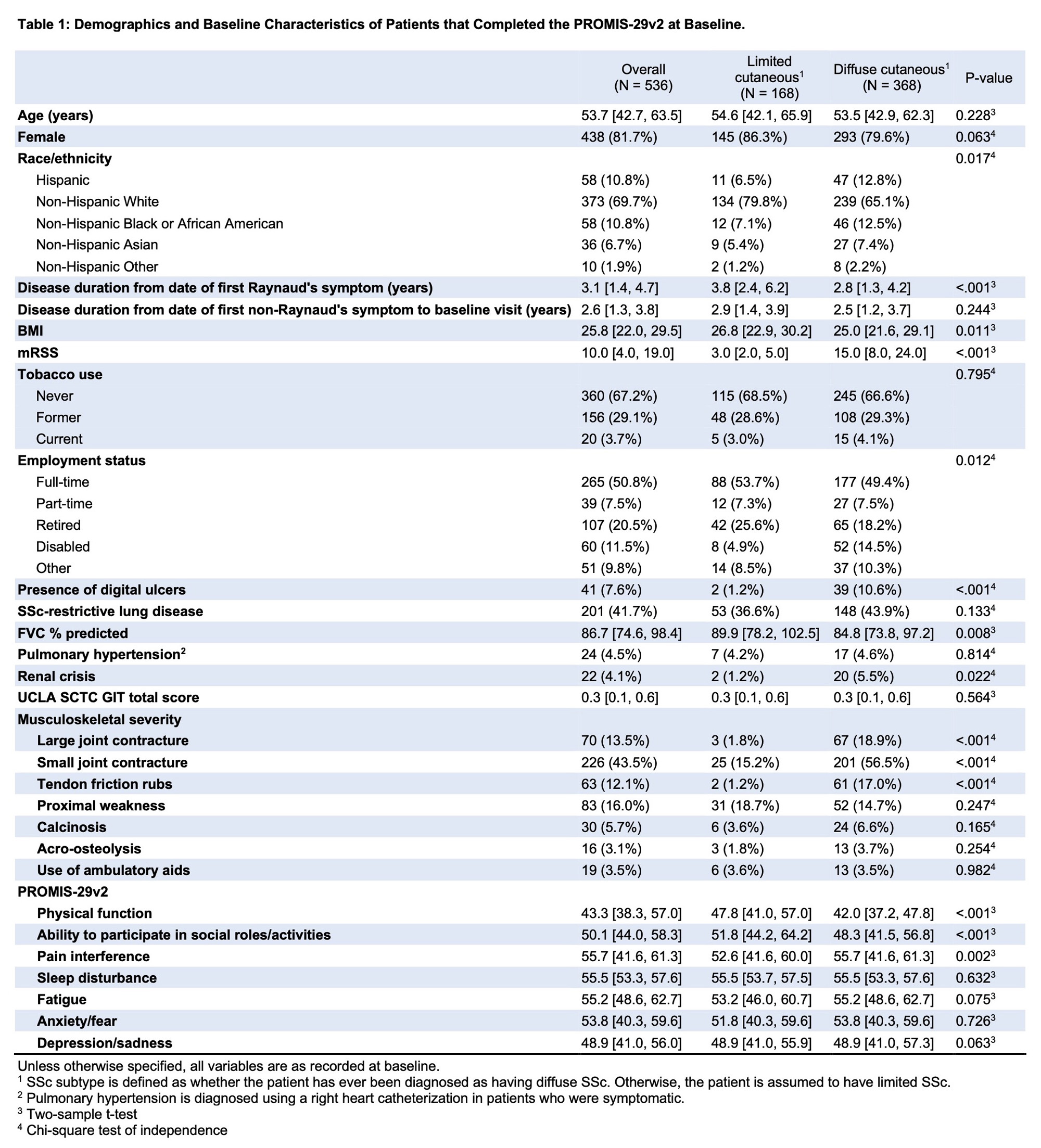
Background/Purpose: Patient-Reported Outcomes Measurement Information System-29 version 2.0 (PROMIS-29v2) is a patient-centered questionnaire used to assess health-related quality of life (HRQoL). PROMIS-29v2 has shown validity and responsiveness to change in patients with systemic sclerosis (SSc) but has not yet been described specifically in early SSc. This study’s aim was to describe PROMIS-29v2 outcomes and clinical associations in the Collaborative National Quality and Efficacy Registry (CONQUER), which is a multi-center US-based registry of patients with early limited cutaneous (lc) and diffuse cutaneous (dc) SSc.
Methods: Patients enrolled in CONQUER meet 2013 ACR-EULAR Classification criteria for SSc and have a disease duration of less than 5 years from their first non-Raynaud SSc symptom. Baseline results of the PROMIS-29v2 domains of physical function (PF), ability to participate in social roles/activities or social function (SF), pain interference, fatigue, anxiety/fear, sleep disturbance, and depression were described for the entire CONQUER cohort and compared between patients with lcSSc and dcSSc using two-sample t-tests. Pearson’s correlation assessed the relationship between patient clinical characteristics and PROMIS-29v2 domain scores.
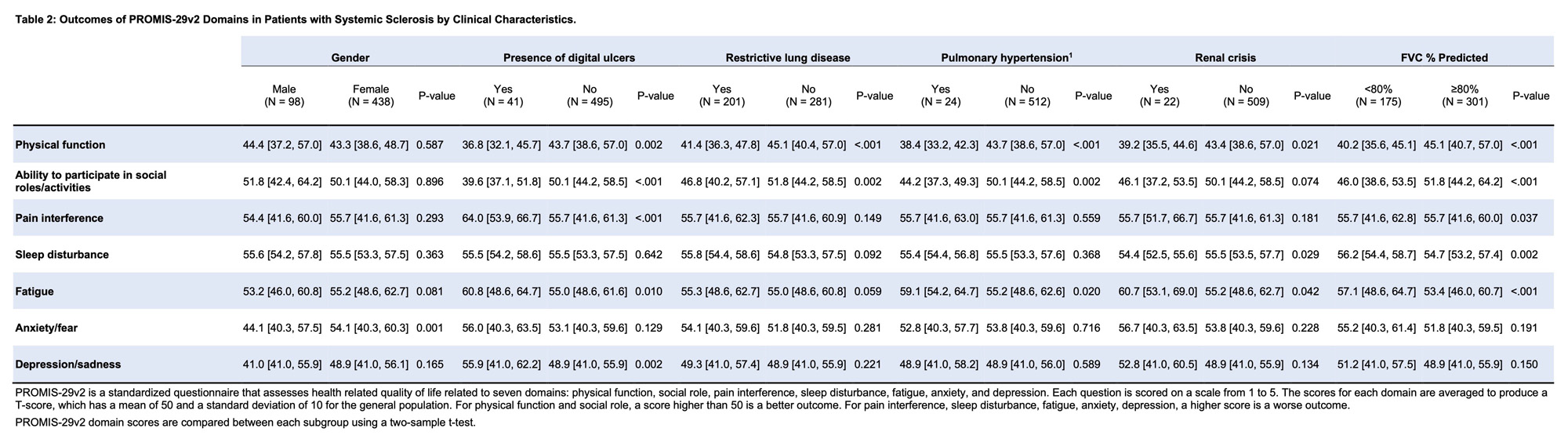
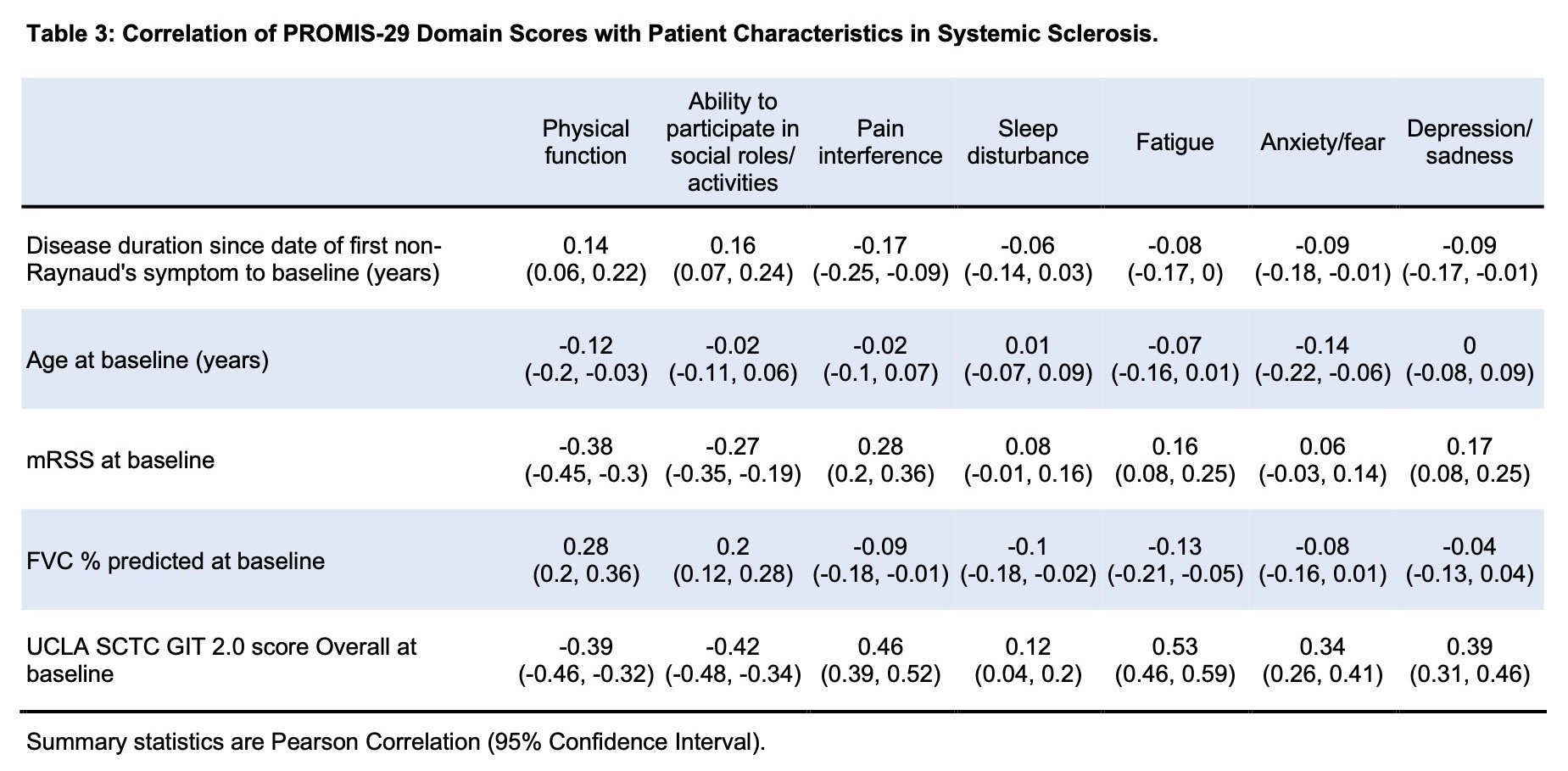
Results: Baseline data were available for 536 participants. Of these, 368 (68.7%) had dcSSc (Table 1). Disease duration from first non-Raynaud’s symptom was 2.6 [1.3, 3.8] years for the whole group and was not significantly different between lcSSc and dcSSc. Individuals with dcSSc had worse PF, SF and pain interference, but significant differences were not seen for fatigue, anxiety/fear, sleep disturbance and depression (Table 1). Female participants had significantly higher anxiety/fear, 54.1 (95% CI 40.3, 60.3) versus 44.1 (40.3, 57.5) for males, p=0.001 (Table 2). PF score was worse (lower) in participants with digital ulcers, restrictive lung disease (RLD, defined as FVC < 70% or TLC < 80%) and pulmonary hypertension. The presence of digital ulcers correlated with worse scores in multiple domains, including PF, SF, pain interference and depression/sadness. RLD correlated with worse PF and SF. Higher mRSS was weakly correlated with PF, r = -0.38 (95% CI -0.45, -0.3) and SF, r = -0.27 (-0.35, -0.19) in the whole group and in the dcSSc group (Table 3). Statistically significant correlations of variable strengths were seen with the UCLA SCTC GIT 2.0 score in all domains (Table 3). The strongest correlations for GIT were seen with fatigue, r = 0.53 (0.46, 0.59); pain interference, r = 0.46 (0.39, 0.52), and SF, r = -0.42 (-0.48, -0.34). Musculoskeletal manifestations including the presence of large or small joint contractures, tendon friction rubs, proximal muscle weakness, calcinosis and the use of ambulatory aids were associated with worse scores in multiple domains (data not shown).
Conclusion: We describe the use of PROMIS-29v2 to assess quality of life in an early US-based SSc cohort. Diffuse disease, higher mRSS, musculoskeletal manifestations, presence of digital ulcers, RLD and UCLA SCTC GIT scores were associated with reduced HRQoL as measured by PROMIS-29v2. Multivariable analysis and assessment of longitudinal change are underway.
To cite this abstract in AMA style:
Lamba I, Makol A, Khanna D, VanBuren J, Child A, Alvey J, Assassi S, Bernstein E, Castelino F, Chung L, Evnin L, Frech T, Hant F, Hummers L, Lakin K, Lebiedz-Odrobina D, Luo Y, Molitor J, Moore D, Richardson C, Sandorfi N, Shah A, Shah A, Shanmugam V, Skaug B, Steen V, Volkmann E, Gordon J. Physical and Mental Health in Early Systemic Sclerosis: Baseline Results for Patient-Reported Outcomes Measurement Information System-29 from the Collaborative National Quality and Efficacy Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/physical-and-mental-health-in-early-systemic-sclerosis-baseline-results-for-patient-reported-outcomes-measurement-information-system-29-from-the-collaborative-national-quality-and-efficacy-registry/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/physical-and-mental-health-in-early-systemic-sclerosis-baseline-results-for-patient-reported-outcomes-measurement-information-system-29-from-the-collaborative-national-quality-and-efficacy-registry/