Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Interstitial lung disease (ILD) is a leading cause of morbidity and mortality in connective tissue disorders (CTD) including rheumatoid arthritis, scleroderma and inflammatory myopathies. Conventional immunosuppressive therapies for CTDs are not always successful at preventing and treating pulmonary interstitial. Mesenchymal Stem Cells (MSC) have immunomodulating properties by blocking T cell proliferation, NK-Cell function and cytokine production. MSC have ability to induce tissue repair. Pulmonary fibrosis maybe the result of failed epithelial repair on stromal cells. Phase 1 trials in patients with post-transplant bronchiolitis obliterans, and idiopathic pulmonary fibrosis have shown MSCs are safe and patients showed stability. These characteristics suggest MSC may be an attractive therapeutic alternative in ILD associated with CTD. In this study, we assessed the safety of the adding allogeneic bone marrow derived mesenchymal stem cells to conventional pharmacological treatment in CTD patients who developed ILD.
Methods: Subjects with CTD who have developed ILD within the previous 2 years, were recruited from the clinical practice of the Pulmonary and Rheumatology clinics in Mayo Clinic Florida. Allogeneic MSC were manufactured at the Mayo Clinic Florida. 0.5 – 1 million MSC per Kg were infused IV. There were no adverse reactions reported during the infusions. Basic CBC and chemistries were obtained pre and post infusion, and on day1, 7, 30, 91 and 182. B, NK and T cell enumerations including T-reg cells, pro-inflammatory cytokines (TH1) and tolerogenic cytokines (TH2) levels as well as pro-angiogenic factors such as VEGF and iNOS were obtained prior to infusion. We assesed. Chest CT without contrast were obtained 1 to 7 days pre-infusion and day 182 post-infusion approximately. Spirometry was obtained at day 1, 7 and 30. Pulmonary function tests were obtained before the infusion, and at day 90 and 182
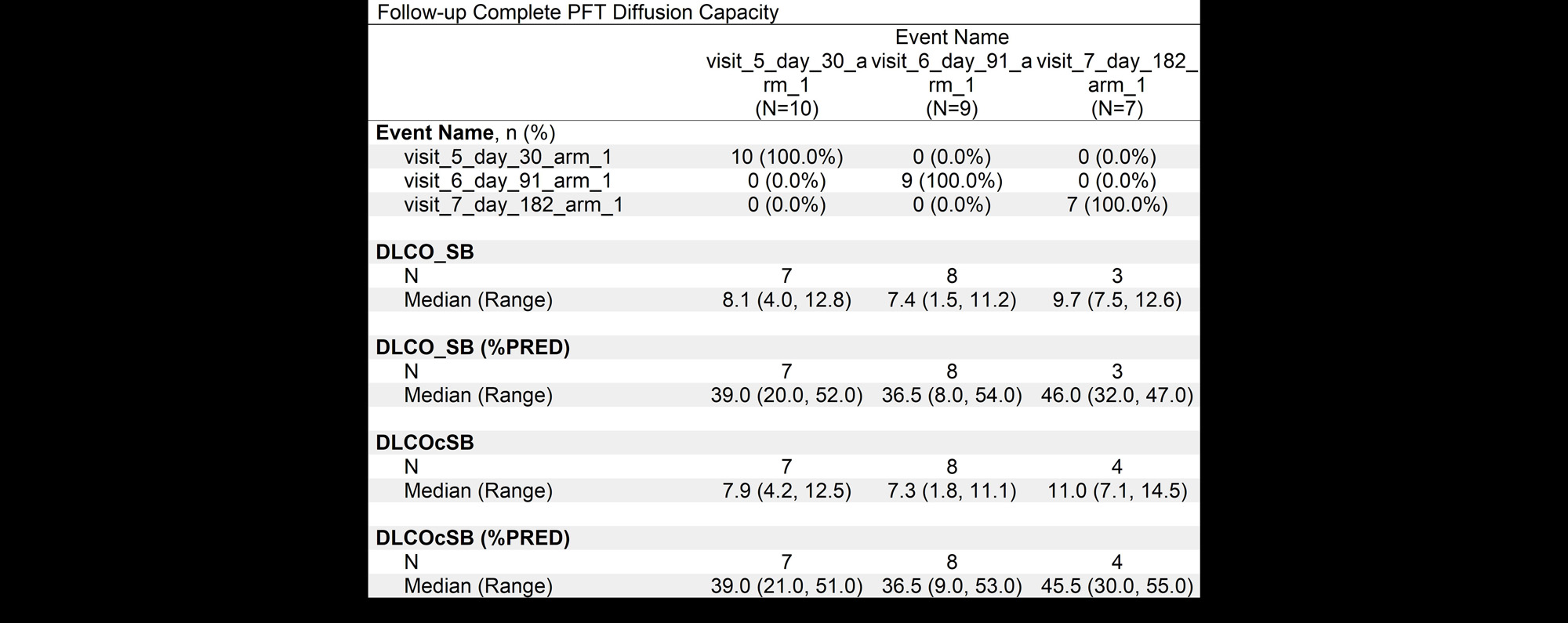
Results: Age ranged from 39-76 years, 7 patients were female and 3 were male.3 patient’s had scleroderma, 3 rheumatoid arthritis, 2 anti synthetase syndrome, 1 polymyositis and 1 patient had interstitial pneumonia with autoimmune features (IPAF). 6 patients had a fibrotic NSIP (Nonspecific Interstitial Pneumonia) pattern, three patients UIP (Usual Interstitial Pneumonia) and 1 patient had fibrotic organizing pneumonia. There were no adverse events related to the MSC infusions. CT scans stable 6 months post-infusion, and in 1 patient the CT worsened. Pulmonary function tests were stable in 4 patients, 4 patients improved and 1 worsened at 6 months post infusion. The 6-minute walk testing showed improved oxygenation in 3 patients, stable numbers in 5 patients and worsening in 2 patients, overall there was a decline on the a distance walked at 6 months. There was a general trend towards improvement of the median diffusion capacity.
Conclusion: The results of our study suggest that MSCs are safe in patients with ILD associated with autoimmune rheumatologic disorders. There were no adverse events related to the infusion. Most patients achieved clinical stability regarding pulmonary function test and CT scan however future Phase II trials would be necessary to determine efficacy in this group of patients.
To cite this abstract in AMA style:
Abril A, Mira-Avendano I, Durand N, Baig H, Lee a, Baer M, Zubair A. Phase I Study to Evaluate the Safety of Allogeneic Bone Marrow Derived Mesenchymal Stem Cells for Interstitial Lung Disease in Patients with Connective Tissue Disorders [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/phase-i-study-to-evaluate-the-safety-of-allogeneic-bone-marrow-derived-mesenchymal-stem-cells-for-interstitial-lung-disease-in-patients-with-connective-tissue-disorders/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/phase-i-study-to-evaluate-the-safety-of-allogeneic-bone-marrow-derived-mesenchymal-stem-cells-for-interstitial-lung-disease-in-patients-with-connective-tissue-disorders/