Session Information
Date: Monday, November 13, 2023
Title: (1221–1255) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Belimumab (BEL) is an approved treatment for SLE, in addition to standard therapy. Intravenous (IV) BEL is approved in patients (pts) ≥5 years of age, and subcutaneous (SC) BEL is approved in adults.1 This study aimed to evaluate the pharmacokinetics (PK), pharmacodynamics (PD), and safety of SC BEL in pediatric pts with SLE.
Methods: This was a multicenter, open-label trial (GSK Study 200908; NCT04179032) of pediatric pts (5–17 years of age) with active SLE who received SC BEL, plus standard therapy, over 52 weeks (wks). A 3-weight band dosing regimen was planned to correct for higher exposure expected with low body weight (≥15–< 30 kg: 200 mg/2 wks; ≥30–< 50 kg: 200 mg/10 days; ≥50 kg: 200 mg/wk). Pts received SC BEL for the first 12 wks (Part A) and could then choose to enter a 40-wk extension (Wk 12–52; Part B). Primary outcome was PK; a population PK (popPK) model (2-compartmental with 1st order absorption, distribution, and elimination) was developed using data from the pediatric IV BEL PLUTO trial (NCT01649765)2 and the current trial, and was informed by a previous model developed for IV BEL in pediatrics.3 Exposures were compared with those in the adult BLISS-SC trial (NCT01484496).4 Secondary outcomes: safety (adverse events [AEs], serious AEs [SAEs], and AEs of special interest [AESI]); PD (percentage change from baseline [BL] in biomarker levels).
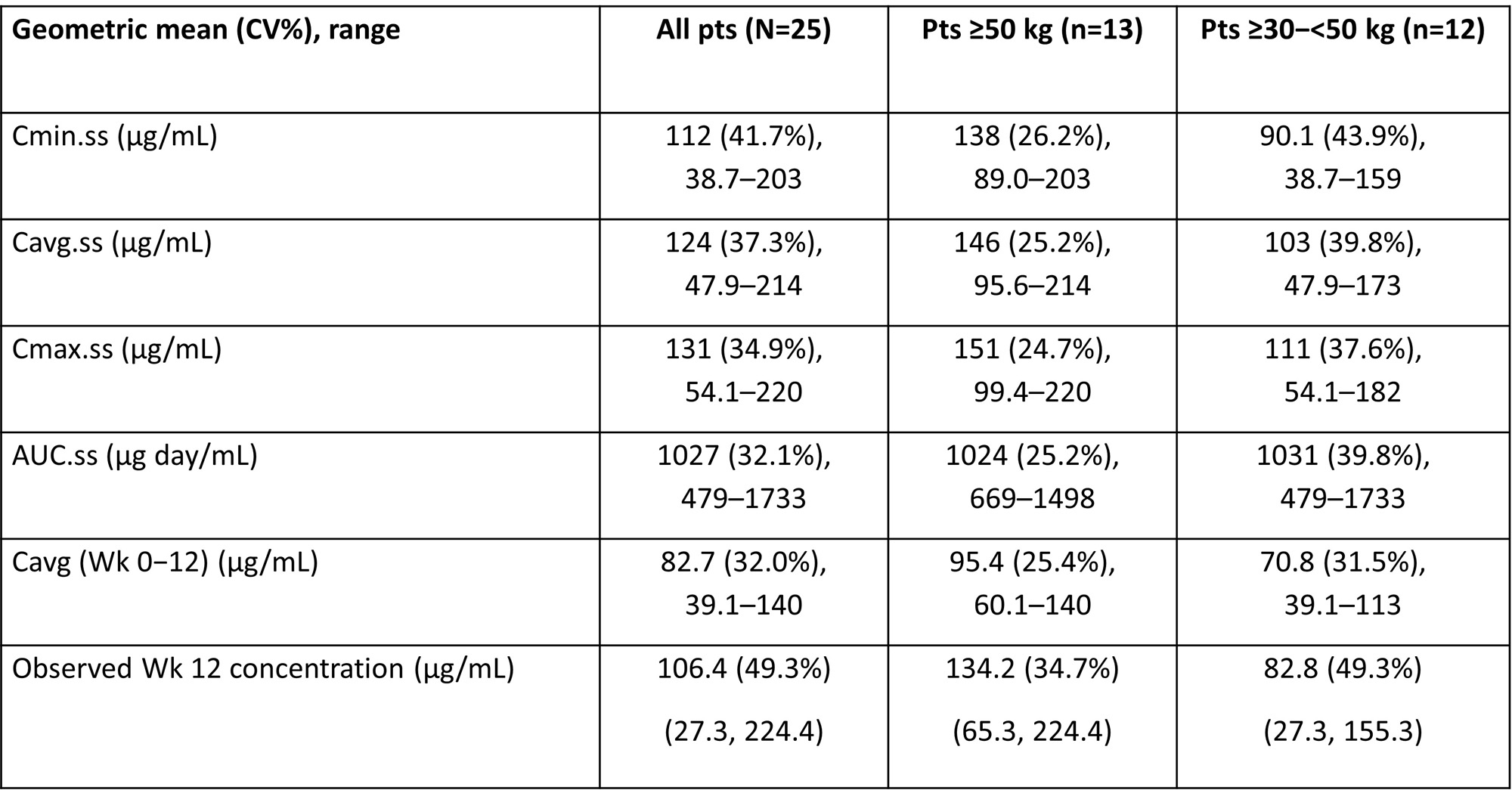
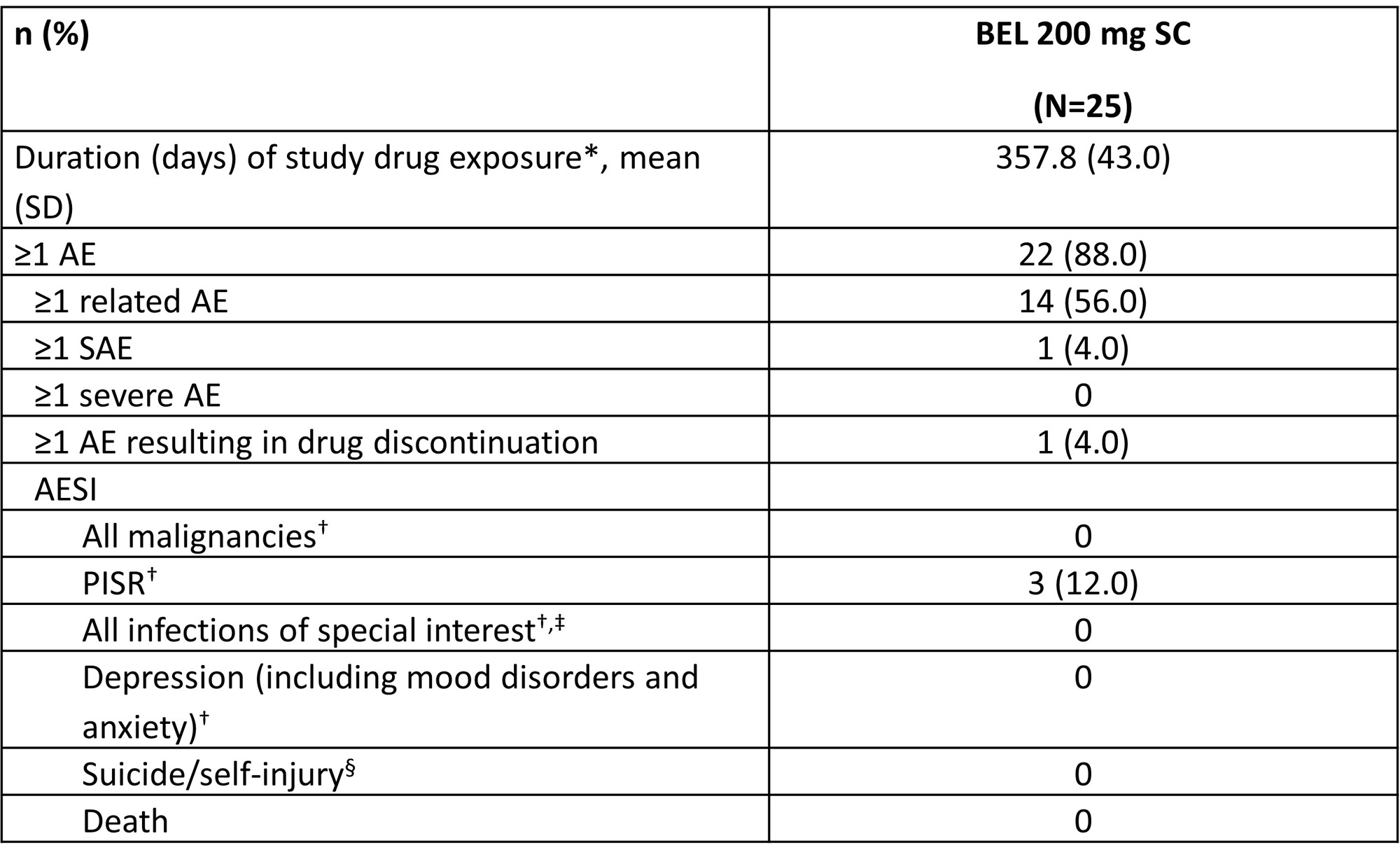
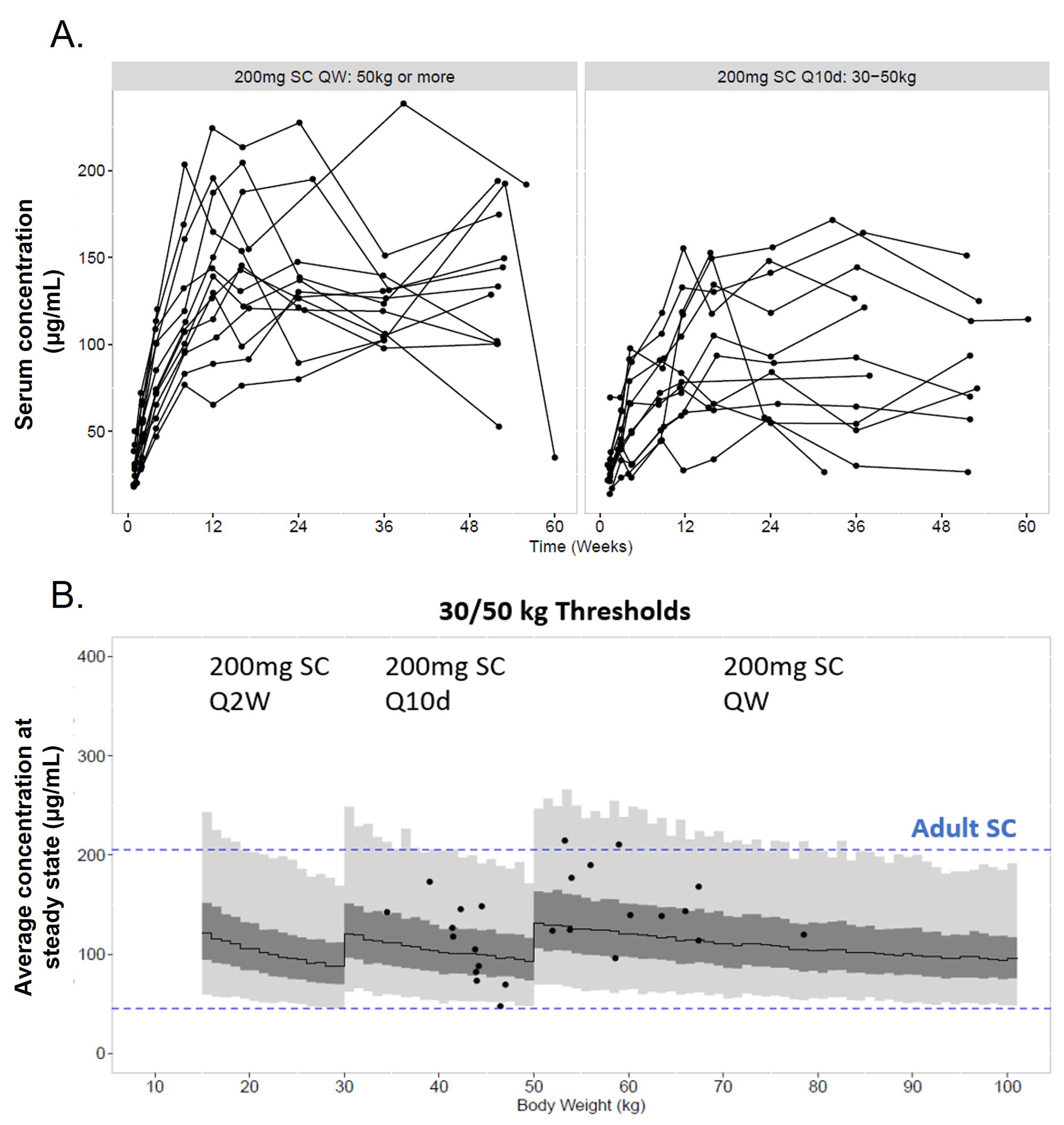
Results: Of the 25 pediatric pts enrolled, 84.0% were female; mean (standard deviation, SD) age at screening was 14.0 (2.1) years. Approximately half of pts were ≥50 kg (52.0%) and 48.0% were ≥30–< 50 kg; no pts were enrolled in the < 30 kg cohort. All pts completed Part A and continued into Part B; 92.0% of pts completed Part B. Mean BEL concentrations were generally higher in pts ³50 kg compared with ³30 to < 50 kg (Table 1, Figure A). However, across a pediatric population, the popPK analysis showed that exposures for the 3-weight band SC regimen were expected to be consistent with adult SC exposures from BLISS-SC (Figure B). Most pts had ≥1 AE (88.0%; Table 2). Nine (36.0%) pts had mild or moderate COVID-19. One (4.0%) pt had a single SAE (mild COVID-19) and was hospitalized, and 3 (12.0%) had an AESI (all post-injection systemic reaction). No infections of special interest, malignancies, depression/suicide/self-injury, or deaths were reported. From BL to Wk 52, there were median percent decreases in Ig levels (IgA: −13.1%; IgG: −12.3%; IgM: −30.8%) and anti-dsDNA antibody levels (−58.9% among those positive at BL). In pts with low complement (C) at BL, there were median percent increases in C3 (+23.5%) and C4 (+67.5%) levels at Wk 52.
Conclusion: SC BEL exposure in pediatric pts with SLE was consistent with the adult SC (200 mg/wk) population. The PD and safety profiles of SC BEL 200 mg in children 5–17 years of age with SLE were consistent with the known profiles of BEL in SLE. No new safety concerns were identified in this population.
Funding: GSK. Authors acknowledge all PLUTO investigators for their contributions.
References
1 Levy RA et al. Lupus 2021;30:1705–21
2 Brunner HI et al. Ann Rheum Dis 2020;79:1340–8
3 Dimelow R et al. Clin Pharmacol Drug Dev 2021;10:622–33
4 Stohl W et al. Arthritis Rheumatol 2017;69:1016–27
AUC.ss, area under the curve at steady state calculated over the dosing period; Cavg.ss, average concentration at steady state; Cavg (Wk 0−12), average concentration over the first 12 wks of treatment; Cmax.ss, maximum concentration at steady state; Cmin.ss, minimum concentration at steady state; CV, coefficient of variation.
*Last injection date − first injection date + X, where X is 7, 10 or 14 for pts ≥50 kg, pts ≥30−50 kg and pts ≥15–<30 kg, respectively. Only complete dates are used. First and last injection dates are used, regardless of any missed doses; †per Custom MedDRA query (version 25.1); ‡includes opportunistic infections, herpes zoster, tuberculosis, and sepsis; §per Standard MedDRA query (version 25.1).
MedDRA, Medical Dictionary for Regulatory Activities; PISR, post-injection systemic reaction.
(B) Individual predicted Cavg of patients (points, N=25); model predicted median (solid line), interquartile range (dark gray) and 95% prediction interval (light gray, and dotted line).
Cavg, average concentration; QW, every week; Q2W, every 2 wks; Q10d, every 10 days.
To cite this abstract in AMA style:
Brunner H, Oscar Viola D, Dimelow R, Calvo Penadés I, Wilkinson C, Cruz Rizo Rodriguez J, Boteanu A, Kamphuis S, Minden K, Horneff G, Anton J, Mori M, Yamasaki Y, Olaiz J, Marino R, van Maurik A, Okily M, Yanni E, WIlde P. Pharmacokinetic, Pharmacodynamic, and Safety Profile of Subcutaneous Belimumab in Pediatric Patients with Systemic Lupus Erythematosus: Analysis of Data from a Multicenter, Open-Label Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/pharmacokinetic-pharmacodynamic-and-safety-profile-of-subcutaneous-belimumab-in-pediatric-patients-with-systemic-lupus-erythematosus-analysis-of-data-from-a-multicenter-open-label-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pharmacokinetic-pharmacodynamic-and-safety-profile-of-subcutaneous-belimumab-in-pediatric-patients-with-systemic-lupus-erythematosus-analysis-of-data-from-a-multicenter-open-label-trial/