Session Information
Date: Sunday, November 13, 2022
Title: Plenary II
Session Type: Plenary Session
Session Time: 11:30AM-1:00PM
Background/Purpose: Knee osteoarthritis (OA) is a heterogeneous disease characterized by a variety of clinical and molecular phenotypes, for which there exist no widely-available biomarkers. We have previously published a pilot analysis of baseline peripheral blood cell DNA methylation patterns as biomarkers of future radiographic progression. In the current study, we apply this method to the FNIH OsteoArthritis Biomarkers Consortium (OABC) subcohort of the OAI including pain-, radiographic-, and dual- (pain+radiographic) progressors compared to nonprogressors and to similarly constructed groups in the Johnston County Osteoarthritis Project (JoCoOA) and an independent OAI cohort from our prior work.
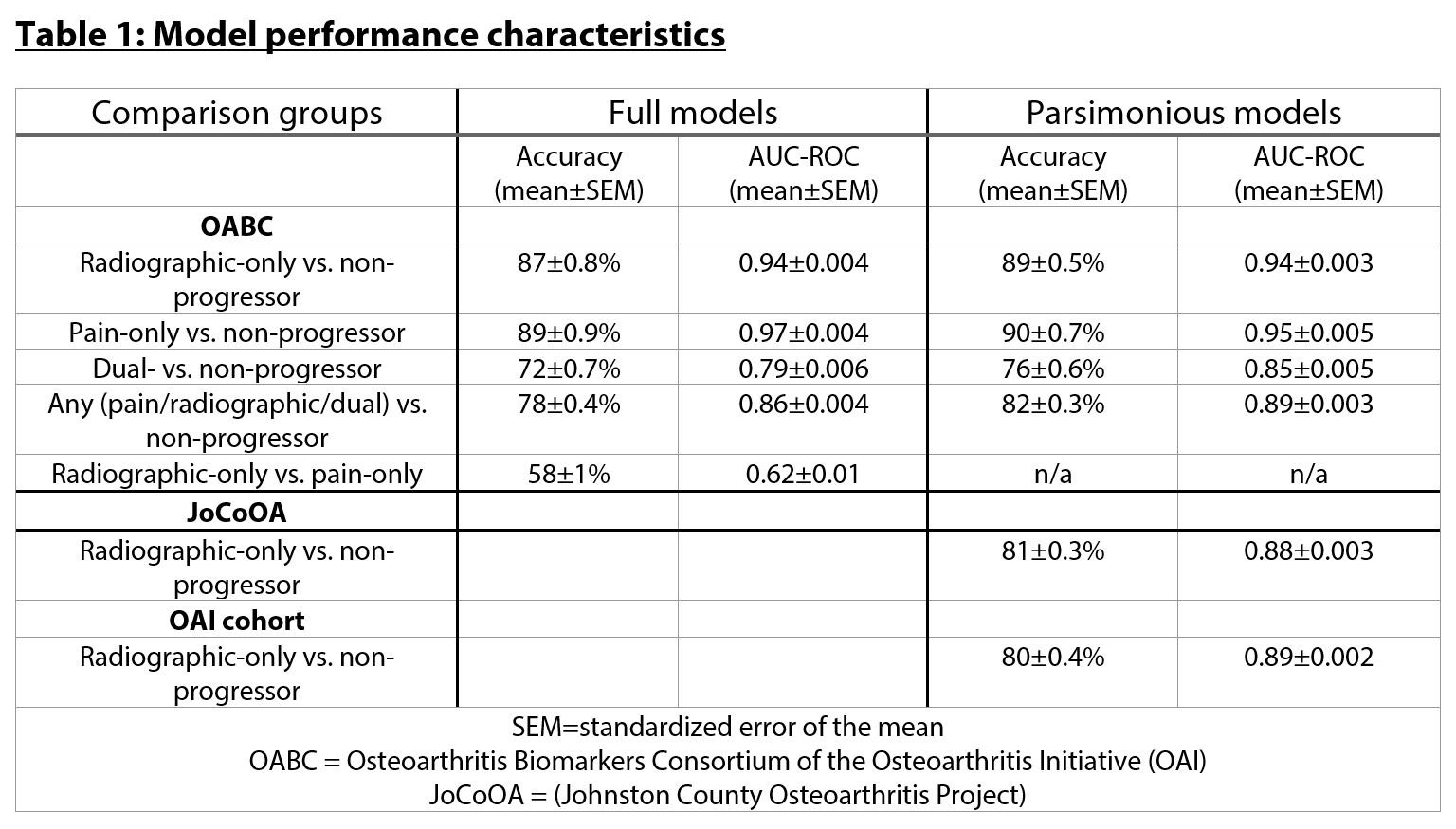
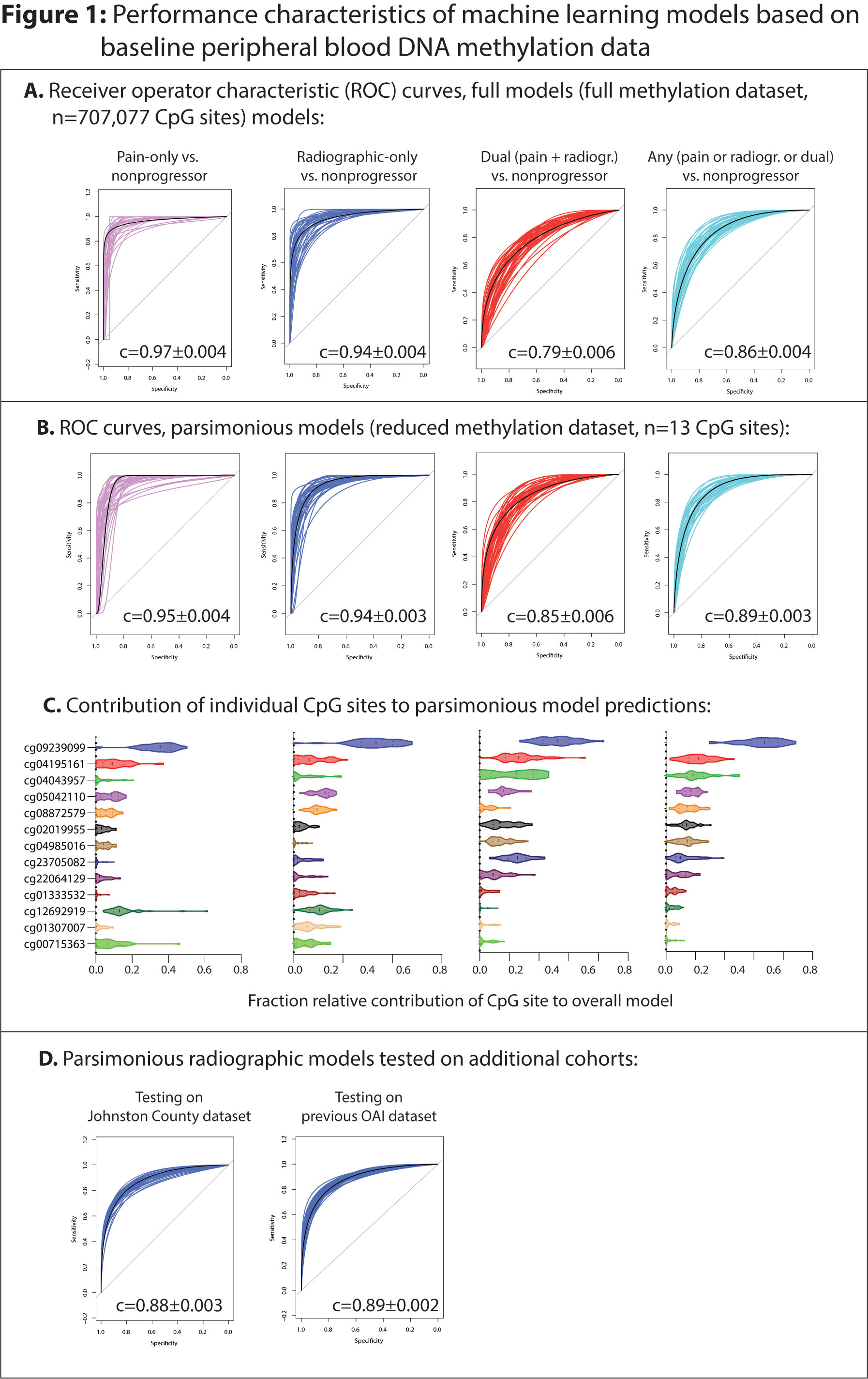
Methods: Buffy coat DNA was obtained from the OAI cohort from baseline visits of OABC participants (n=554). 500ng of DNA was bisulfite treated and loaded onto Illumina EPIC arrays then imaged by the Clinical Genomics Center at the Oklahoma Medical Research Foundation. Raw data were extracted and BMIQ normalized using the ChAMP package. Elastic net-penalized generalized linear models (GLMs) were then developed using 40 cycles of a 70%-development, 30%-validation data split. Parsimonious models were developed by reducing the dataset to include DNA methylation sites selected in ≥10/40 development rounds (n=13 CpGs). Parsimonious models were tested on an independent radiographic progressor validation cohort from the JoCoOA, including 85 future progressors (≥1 K/L grade worsening at 48 mo or joint replacement) and 56 non-progressors (no- or within-grade K/L worsening) and on DNA methylation data from our previous peripheral blood methylation work including an indepdent set of 27 radiographic-only and 28 non-progressors within the OAI.
Results: Baseline buffy coat DNA methylation patterns accurately predicted future radiographic (accuracy 87±0.8% mean±standard error of mean [SEM], Table 1), pain (89±0.9%), dual radiographic+pain (72±0.7%), and ‘any’ progressors (radiographic, pain, or dual, 78±0.4%). Intriguingly, pain-only and radiographic-only progressors were not distinguishable (accuracy 58±1%). The inclusion of demographic characteristics or baseline serum/urine analytes did not alter model performance. Parsimonious models including the top 13 CpG sites selected during full development had similar accuracy. Despite differences in the definition of radiographic progression, models still accurately discriminated radiographic progressors from non-progressors in both the JoCoOA (81±0.3%) and OAI (80±0.3%) validation cohorts.
Conclusion: Herein, we evaluated the predictive capability of peripheral blood-based DNA methylation models in a large cohort of participants with OA (OABC) and confirmed these findings in two independent cohorts. Our data suggest that pain and structural progression share similar early systemic immune epigenotypes. Further work should focus on evaluating the pathophysiological consequences of differential DNA methylation of peripheral blood cell subtypes in individuals with knee OA.
To cite this abstract in AMA style:
Dunn C, Sturdy C, Velasco C, Schlupp L, Prinz E, Izda V, Arbeeva L, Golightly Y, Nelson A, Jeffries M. Peripheral Blood DNA Methylation-based Machine Learning Models for Prediction of Knee Osteoarthritis Progression: Biospecimens and Data from the Osteoarthritis Initiative and Johnston County Osteoarthritis Project [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/peripheral-blood-dna-methylation-based-machine-learning-models-for-prediction-of-knee-osteoarthritis-progression-biospecimens-and-data-from-the-osteoarthritis-initiative-and-johnston-county-osteoarth/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/peripheral-blood-dna-methylation-based-machine-learning-models-for-prediction-of-knee-osteoarthritis-progression-biospecimens-and-data-from-the-osteoarthritis-initiative-and-johnston-county-osteoarth/