Session Information
Date: Saturday, November 16, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
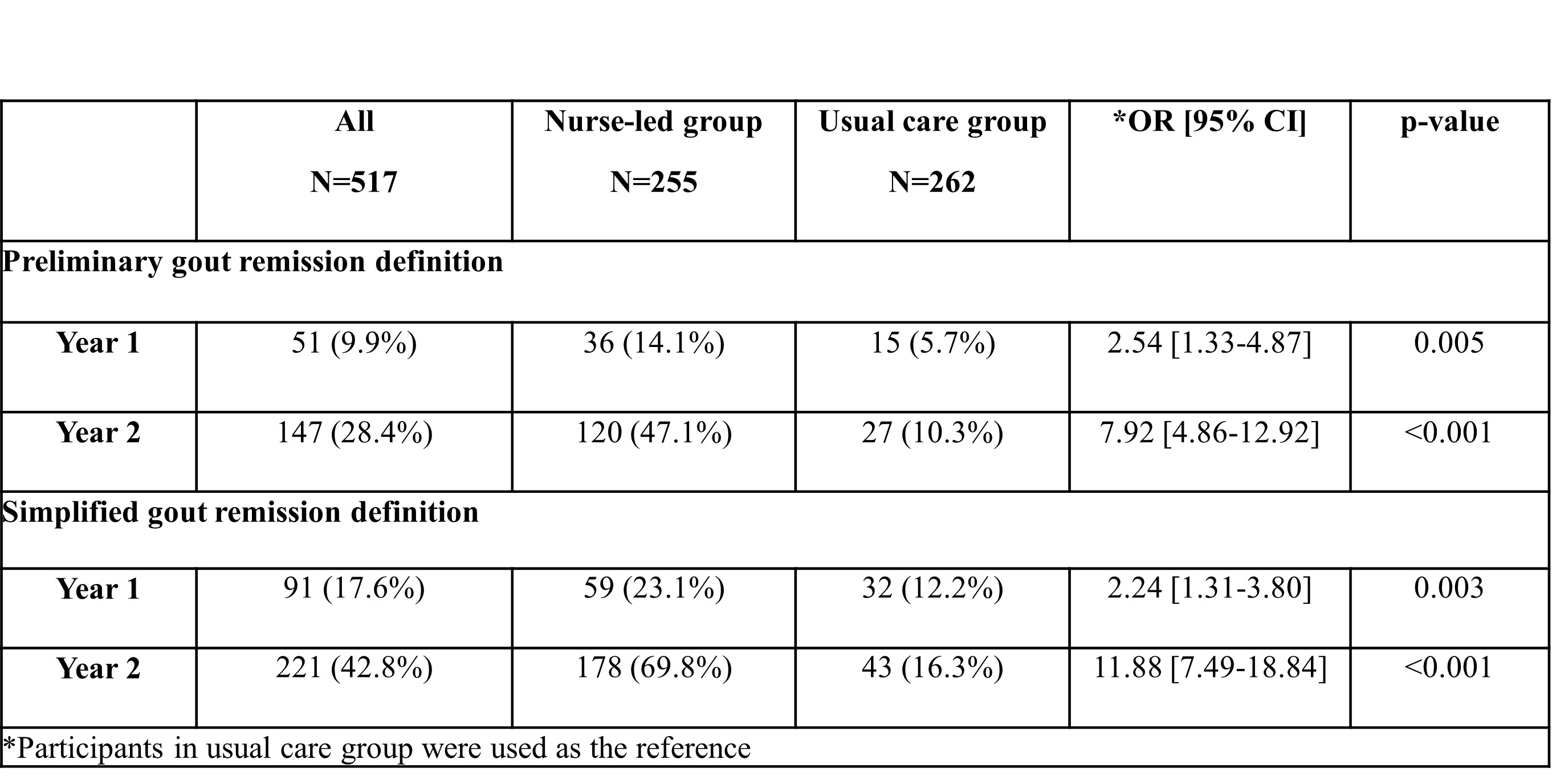
Background/Purpose: To compare the performance of the 2016 preliminary gout remission definition and a simplified gout remission definition in a clinical trial of nurse-led gout care.
Methods: Data were analyzed from a 2-year parallel arm, non-blinded, randomised controlled trial of 517 people with gout, in which participants were assigned 1:1 to receive nurse-led care or usual care1. Remission was defined using the 2016 preliminary gout remission definition2 and a simplified gout remission definition (without serial measures of patient global assessment and pain scores). Binary logistic regression was used to compare intervention groups. Gout-specific quality of life was assessed using items from the Gout Impact Scale (GIS), measured on a 0-100 scale. General linear models were used to compare GIS scores between those in remission and those not in remission using either definition.
Results: During the trial participants in the nurse-led group were more likely to achieve remission using either definition; at Year 2 the odds ratio was 7.92 [95% CI 4.86-12.92] using the 2016 preliminary definition and 11.88 [95% CI 7.49-18.84] using the simplified definition (Table 1). For all participants, remission was more common using either definition in Year 2 than Year 1 (p< 0.001), and at both Year 1 and Year 2 more participants were defined as being in remission using the simplified definition compared with the 2016 preliminary definition (p< 0.001). People in remission using either definition had better gout outcomes assessed using the GIS, including greater control over their gout, with a mean difference of 9.85 [95% CI 3.95-15.74], p< 0.001 using the preliminary definition, and a mean difference of 9.87 [95% CI 3.72-16.01], p= 0.002 using the simplified definition.
Conclusion: The 2016 preliminary gout remission definition and the simplified gout remission definition both discriminated well between nurse-led and usual care groups. The simplified definition identified more people in both groups as being in gout remission and showed high construct validity using a gout-specific health related quality of life instrument. The simplified definition is a feasible and valid option for defining gout remission in gout clinical trials.
1. Doherty et al, Lancet 2018;392:1403-12
2. de Lautour et al, Arthritis Care Res 2016;68:667-72
To cite this abstract in AMA style:
Tabi-Amponsah D, Doherty M, Sarmanova A, Zhang W, Stewart S, Taylor W, Stamp L, Dalbeth N. Performance of Two Gout Remission Definitions in a Two-Year Randomized Controlled Trial of Nurse-led Care [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/performance-of-two-gout-remission-definitions-in-a-two-year-randomized-controlled-trial-of-nurse-led-care/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-of-two-gout-remission-definitions-in-a-two-year-randomized-controlled-trial-of-nurse-led-care/