Session Information
Date: Friday, March 31, 2023
Title: Poster Breakout 4 - JDM & Scleroderma: Clinical & Therapeutic Aspects
Session Type: Breakout Session
Session Time: 4:30PM-5:00PM
Background/Purpose: Gastrointestinal (GI) manifestations in juvenile onset systemic sclerosis (jSSc) reflect adult disease with a range of involvement along the GI tract, including oropharyngeal dysphagia and intestinal dysmotility, which lead to malnutrition (15-56% jSSc) and increased mortality. Patient reported outcomes (PRO) to capture the impact of GI disease in children would be useful in detecting underlying GI problems and to establish outcome measures that capture change, optimizing understanding of therapeutic impact. The UCLA Scleroderma Clinical Trial Consortium Gastrointestinal Tract 2.0 (UCLA-GIT 2.0; GIT) is one of the most widely used and validated PRO for severity of GI involvement in adult-onset SSc (aSSc). To our knowledge the GIT 2.0s performance in jSSc has not been established.
Methods: All jSSc subjects enrolled in the National Registry for Childhood Onset Scleroderma, a prospective observational clinical research registry at a multi-disciplinary center, with a GIT collected at study visit were included. Demographic, clinical, and PRO data of interest were extracted. Summary statistics were applied to outcome measures. Spearman correlation coefficient was applied to analyze relationships between the GIT total and subscales to a traditional global GI involvement PRO, the intestinal visual analog scale of the Scleroderma Health Assessment Questionnaire (SHAQ-GI-VAS) for convergent construct validity (significance defined as p 0.05).
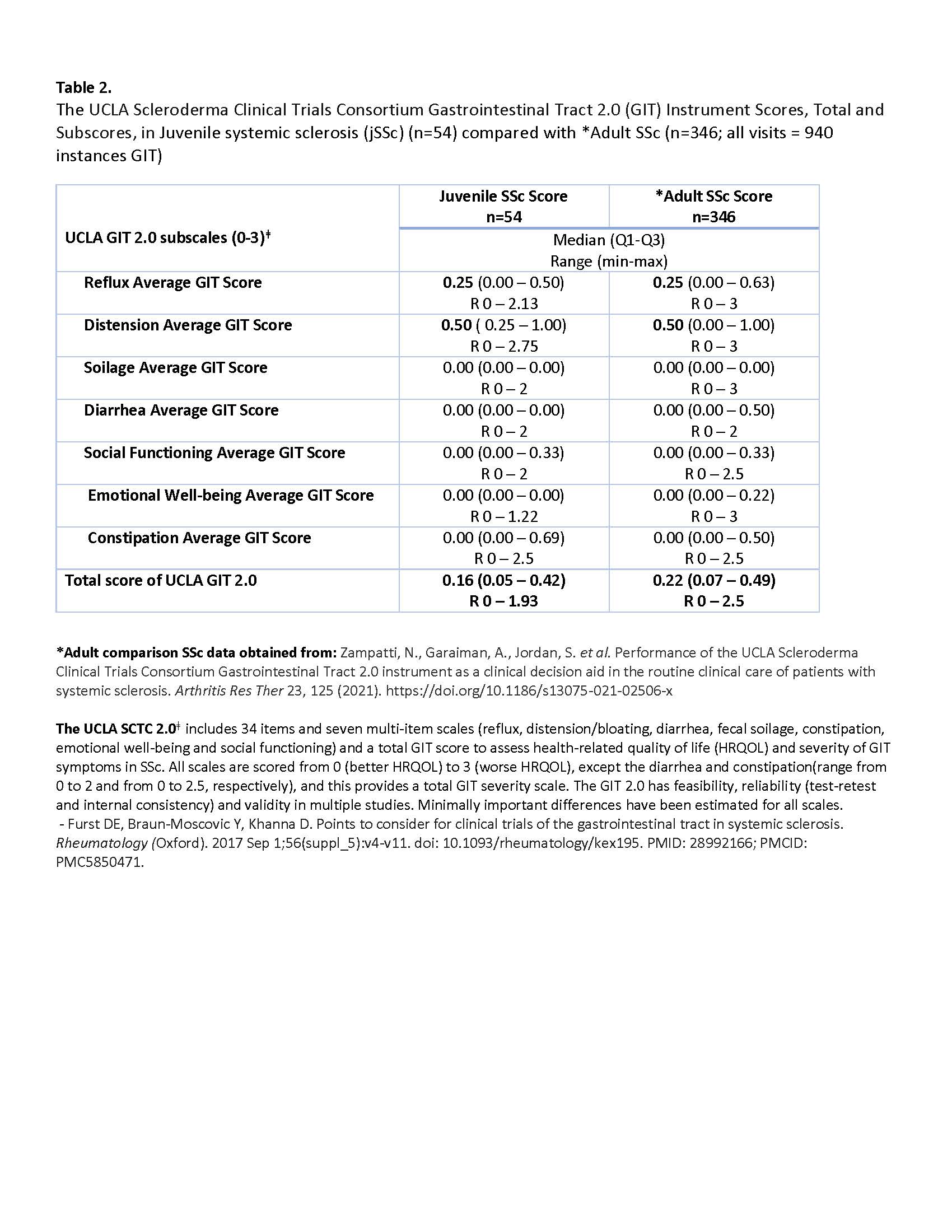
Results: Data for a total of 54 patients with jSSc were extracted. The average age of onset was 9.9 years old, and there was 3.3 years disease duration at time of initial GIT collection. Demographics, autoantibody, classification and clinical variables are summarized in Table 1. The median (IQR) of the GIT 2.0 and its subscales in our jSSc cohort are displayed in Table 2, adjacent to a published adult comparison cohort. Most values are comparable to those found in adult SSc subjects with Distension and Reflux having the most impact. The GIT and its subscales correlated well with the SHAQ-GI-VAS scale, with the Total score having the strongest association, and notable moderate correlations with Social functioning and Emotional well-being (Table 3). Diarrhea was the only subscale not to correlate with SHAQ-GI-VAS. In addition, the Total score also correlated moderately to the SHAQ Global overall Disease impact (rs 0.64, p 0.001).
Conclusion: Results from a single center jSSc cohort demonstrate the GIT 2.0 is likely a useful tool in pediatric scleroderma, despite its development in adult disease. The literature finds the median Total score and subscale scores in adults to be similar to those reported here in jSSc, with greatest impact of GI issues to be from Distention and Reflux domains. Targeting supportive and pharmacological therapy in these areas should be pursued. This study also supports the social and emotional impact of GI disease using a PRO in pediatrics, which is important as patients (teens especially) are reluctant to fully answer GI related questions directly from the physician. The next step is to evaluate the GITs sensitivity to change in jSSc via longitudinal study visits in relation to GI and other outcomes.
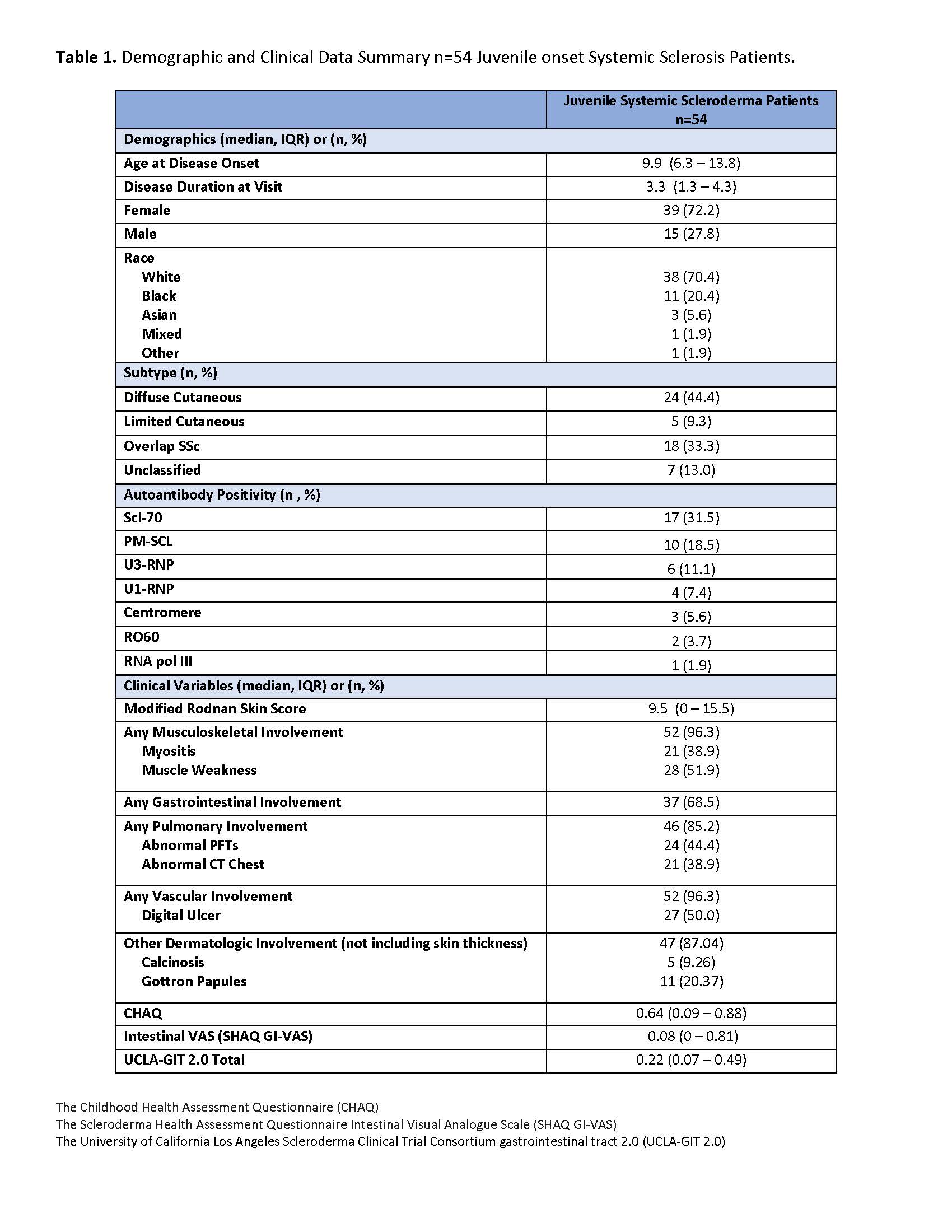 Table 1. Demographic and Clinical Data Summary n=54 Juvenile onset Systemic Sclerosis Patients.
Table 1. Demographic and Clinical Data Summary n=54 Juvenile onset Systemic Sclerosis Patients.
The UCLA Scleroderma Clinical Trials Consortium Gastrointestinal Tract 2.0 (GIT) Instrument Scores, Total and Subscores, in Juvenile systemic sclerosis (jSSc) (n=54) compared with *Adult SSc (n=346; all visits = 940 instances GIT)
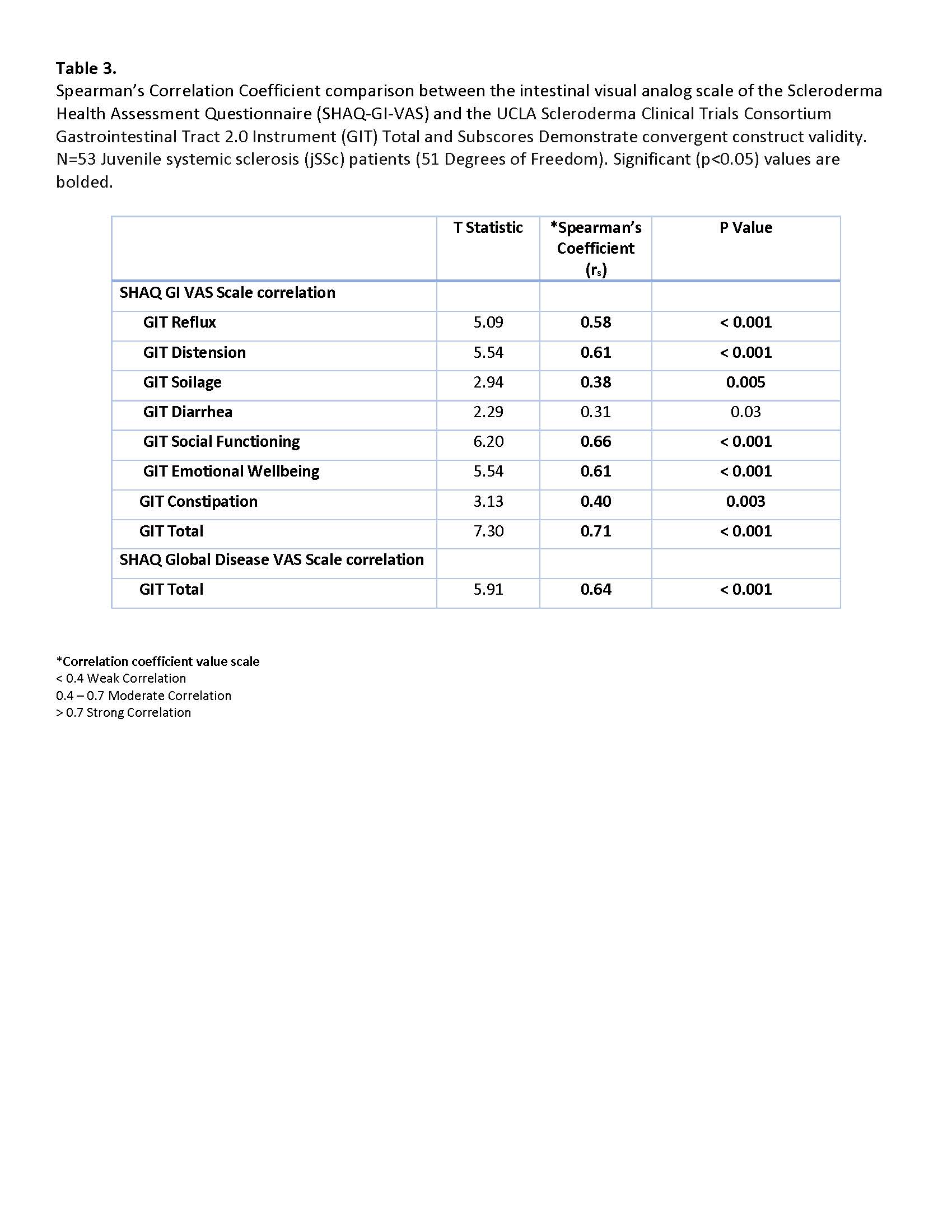 Spearman’s Correlation Coefficient comparison between the intestinal visual analog scale of the Scleroderma Health Assessment Questionnaire (SHAQ-GI-VAS) and the UCLA Scleroderma Clinical Trials Consortium Gastrointestinal Tract 2.0 Instrument (GIT) Total and Subscores Demonstrate convergent construct validity. Nf53 Juvenile systemic sclerosis (jSSc) patients (51 Degrees of Freedom). Significant (p < 0.05) values are bolded.
Spearman’s Correlation Coefficient comparison between the intestinal visual analog scale of the Scleroderma Health Assessment Questionnaire (SHAQ-GI-VAS) and the UCLA Scleroderma Clinical Trials Consortium Gastrointestinal Tract 2.0 Instrument (GIT) Total and Subscores Demonstrate convergent construct validity. Nf53 Juvenile systemic sclerosis (jSSc) patients (51 Degrees of Freedom). Significant (p < 0.05) values are bolded.
To cite this abstract in AMA style:
Stefancic S, Robinson A, Havrilla H, Branton S, Sood V, Torok K. Performance of the UCLA Scleroderma Clinical Trials Consortium Gastrointestinal Tract 2.0 Instrument in a Juvenile Systemic Sclerosis Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/performance-of-the-ucla-scleroderma-clinical-trials-consortium-gastrointestinal-tract-2-0-instrument-in-a-juvenile-systemic-sclerosis-cohort/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/performance-of-the-ucla-scleroderma-clinical-trials-consortium-gastrointestinal-tract-2-0-instrument-in-a-juvenile-systemic-sclerosis-cohort/