Session Information
Date: Monday, November 9, 2020
Title: Pediatric Rheumatology – Clinical Poster III: SLE, Vasculitis, & JDM
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: The value of one-year protocol kidney biopsies in children who have proliferative lupus nephritis (LN) is unclear, particularly in patients who meet CARRA criteria for a complete renal response.
Methods: Subjects < 18 yrs of age, who met ACR systemic lupus erythematosus (SLE) classification criteria with initial kidney biopsy (KBx) proven proliferative LN at Rady Children’s Hospital San Diego (RCHSD) between 1/1/2010 – 12/31/2019 and a pre-planned one year protocol KBx (0.75-1.25 years), were selected for this study. Kidney biopsies were read by RCHSD Pathologists. CARRA criteria for complete response (CR), partial remission (PR) and flare (F) were assessed at the time of protocol KBx. Patients were allocated into either CR or non-complete response (PR and F) groups (NCR) for analysis. UCSD/RCHSD IRB 200870X
Results: Of the 19 patients who had a one year protocol KBx, there were 11 CR (58%) and 8 NCR study subjects. There were no statistically significant differences between CR and NCR patients at initial KBx. Induction therapy was different between the two groups, with CR subjects more likely to receive intravenous cyclophosphamide (CTX). Patients with CR had significantly improved ISN/RPS class and lower NIH activity and chronicity scores on follow up biopsy compared to the NCR group. There was no statistically significant worsening in CR NIH chronicity scores. All SLEDAI scores in CR group at the time of protocol KBx were 4 or less, but 2 NCR patients also had SLEDAI scores of 4. All but one patient in the NCR group had a change (n=4) or increase in maintenance immunosuppression (n=3) after their KBx. Five CR subjects had no change in immunosuppression; while 6 (55%) had a decrease in immunosuppression. CR patients with immunosuppression reduction were more likely to have a decrease of ≥5 in NIH activity scores. There was one perinephric hemorrhage out of 38 KBx (2.6%) that necessitated an overnight hospital stay.
Conclusion: One year protocol KBx were beneficial in most patients with proliferative LN because the KBx provided histopathologic data to support a change in immunosuppression. Subjects meeting the CARRA criteria for complete renal response (CR) at had significantly better one year protocol KBx outcomes with greater ISN/RPS class improvement, and lower NIH activity and chronicity scores. Unexpectedly, two CR patients had KBx that no longer met the criteria for ISN/RPS class 1. CTX induction therapy of proliferative LN may have been a factor leading to better outcomes. While it is not unexpected that immunosuppression was changed or increased in nearly all NCR patients, the improved KBx results in CR subjects created a decision tree, where physicians either: 1) continued the effective immunosuppression, or 2) decreased immunosuppression. One year protocol KBx were associated with a low risk of serious adverse events. Study limitations include: a single center, non-randomized cohort, physician-directed protocol KBx selection and grouping of partial remission and flare groups into the NCR group. A prospective randomized trial would be able to determine if decreasing immunosuppression at one year for CR subjects would yield acceptable outcomes, as assessed by a two year protocol KBx.
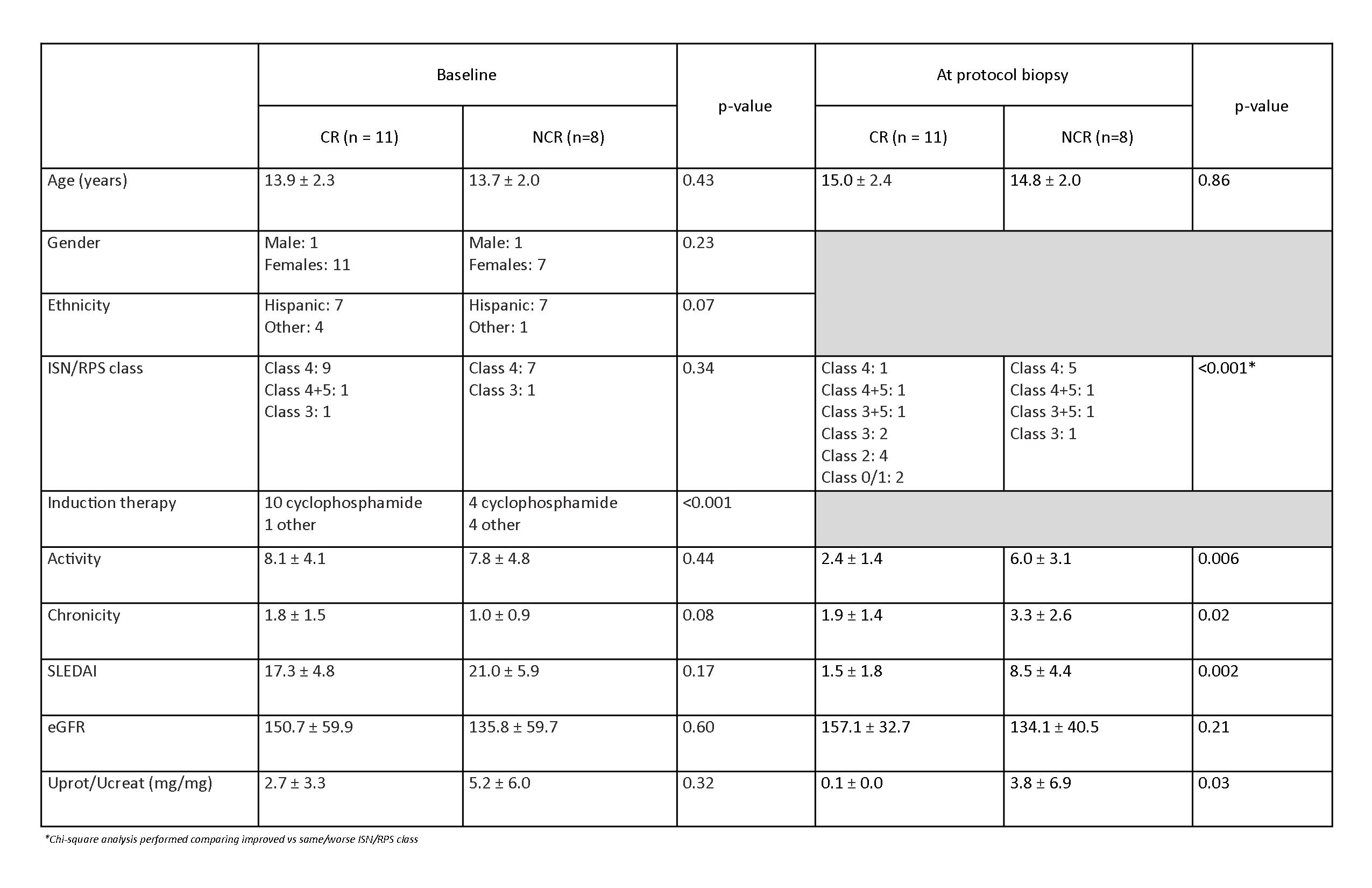 *Chi-square analysis performed comparing improved vs same/worse ISN/RPS class
*Chi-square analysis performed comparing improved vs same/worse ISN/RPS class
To cite this abstract in AMA style:
Yorgin P, Radhakrishna S, Carter C, Chang J, Shayan K, Nguyen L, Chiraseveenuprapund P, Sheets R. Pediatric and Adolescent One Year Protocol Kidney Biopsies Should Be Performed, Even in Patients with Complete Remission of Their Lupus Nephritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/pediatric-and-adolescent-one-year-protocol-kidney-biopsies-should-be-performed-even-in-patients-with-complete-remission-of-their-lupus-nephritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pediatric-and-adolescent-one-year-protocol-kidney-biopsies-should-be-performed-even-in-patients-with-complete-remission-of-their-lupus-nephritis/
