Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: RA is commonly diagnosed through a serological test for the presence of ACPA, and RA patients who test positive are collectively known as ‘ACPA-positive RA’ (ACPA+). However, patients can test negative for ACPA yet still be clinically diagnosed with RA, and are thereby designated as ‘ACPA-negative RA’ (ACPA–). Although ACPA+ and ACPA– patients are known to have different rates of erosive damage and distinct risk factors, the differences in serological profiles between these two disease subgroups are largely unknown. In this study, we performed a comprehensive survey of serum autoantibodies in patients with ACPA+ and ACPA– RA to obtain novel insights into autoantigens underlying the distinct subgroups of RA.
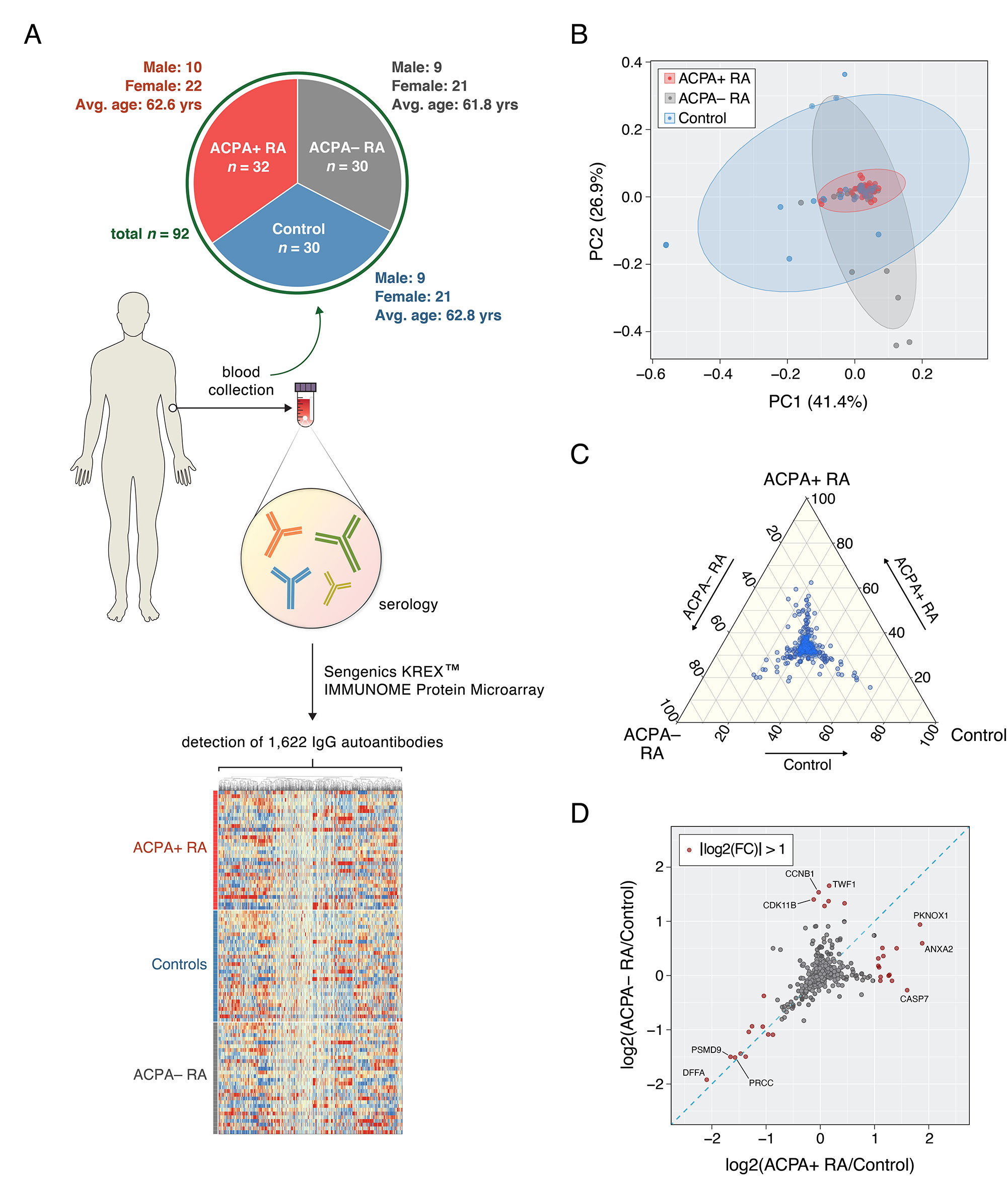
Methods: Serum was collected from patients with ACPA+ RA (n = 32), patients with ACPA– RA (n = 30), and healthy controls (n = 30) (Fig. 1A). Sengenics KREXTM multiplexed autoantibody-screening protein microarray was used to screen for 1,622 different autoantibodies from each serum sample. A Mann–Whitney U test combined with Cliff’s delta was used to identify differentially abundant autoantibodies.
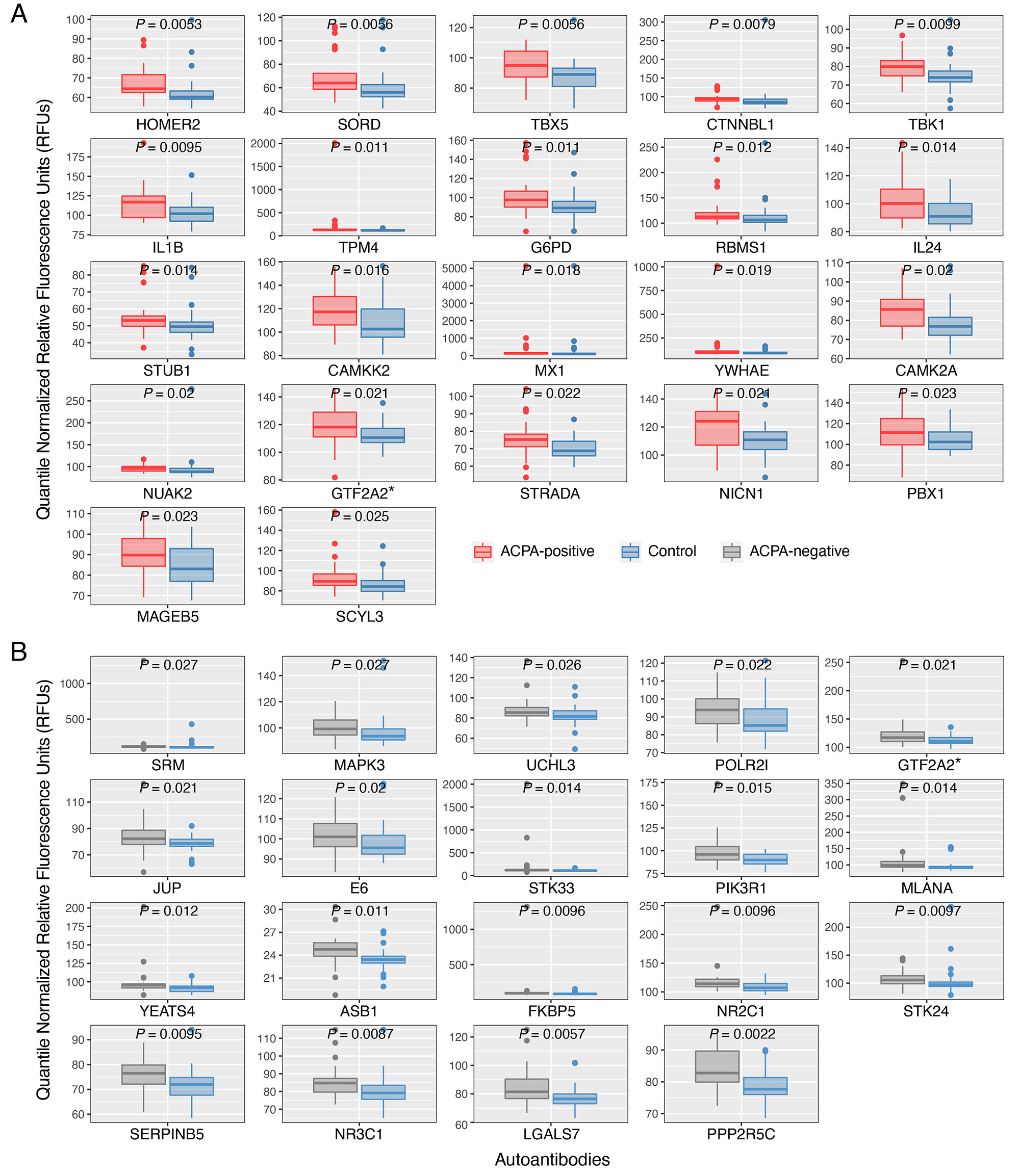
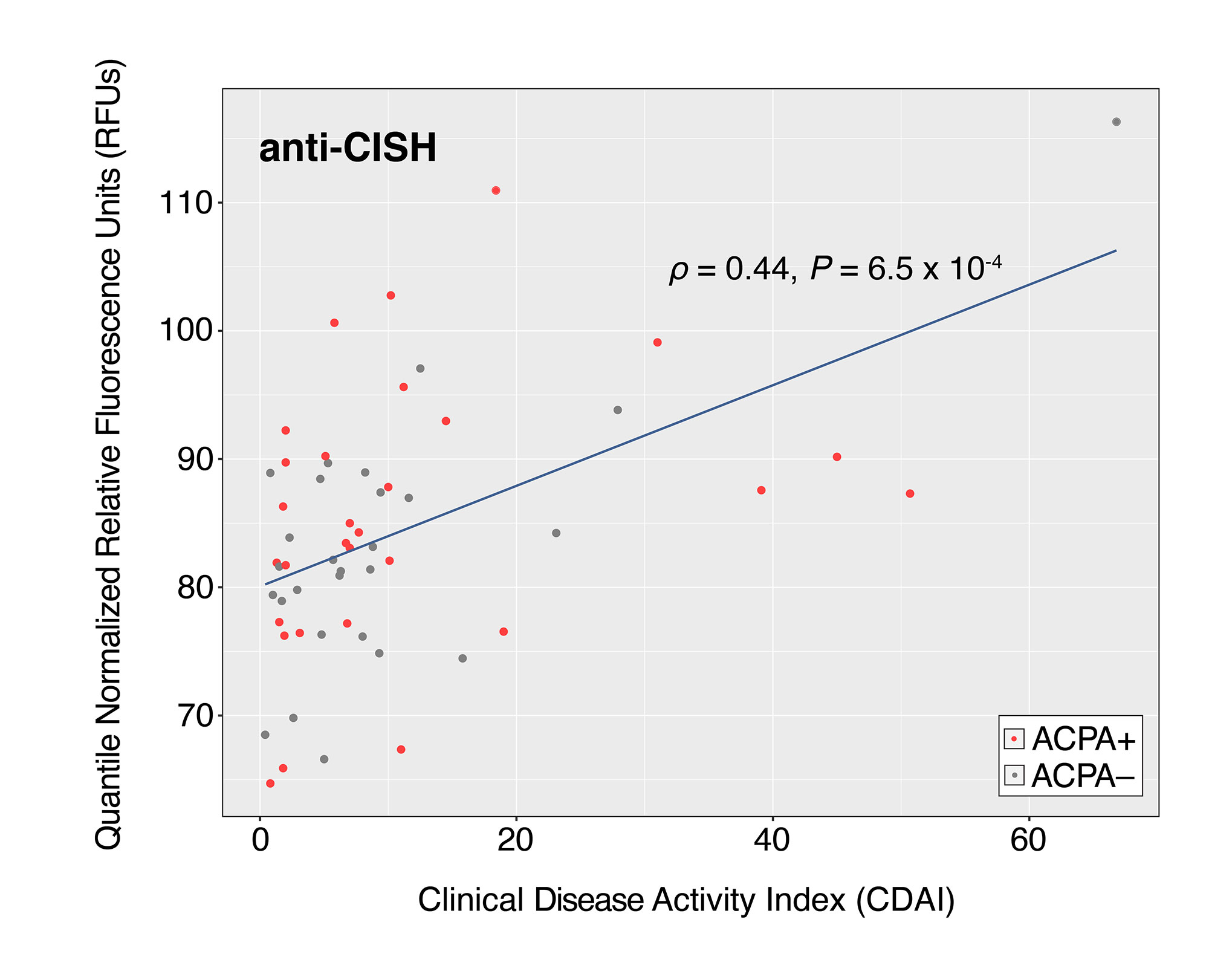
Results: ACPA+ RA patients, ACPA– RA patients, and healthy controls displayed differences in their serum autoantibody profiles (Fig. 1B–D). Upon closer examination, we identified differential serum autoantibodies of ACPA+ and ACPA– RA. Specifically, we found 22 and 19 autoantibodies higher in ACPA+ RA patients and ACPA– RA patients, respectively, compared to healthy controls (Fig. 2A–B). Notably, only one autoantibody for GTF2A2 was found to be higher in both RA subgroups. Next, we found autoantibodies for the CISH protein to be positively correlated with the Clinical Disease Activity Index (ρ = 0.44, P = 6.5 × 10-4) (Fig. 3). Additionally, we determined the functional classes of the proteins targeted by the differentially abundant autoantibodies. We found eight different functional classes, using the PANTHER classification system, for the autoantibodies higher in ACPA+ RA patients, and seven different functional classes for the autoantibodies higher in ACPA– RA patients. When comparing these two groups of functional classes, we identified four in common: ‘Gene-specific Transcriptional Regulator’, ‘Nucleic Acid Metabolism Protein’, ‘Metabolite Interconversion Enzyme’, and ‘Protein-modifying Enzyme’. This suggests that an imbalance in autoantibody production targeting metabolic and transcription processes could potentially be a newly identified hallmark of RA.
Conclusion: We identified several differentially abundant autoantibodies in ACPA+ and ACPA– RA, many of which were identified for the first time. Our study demonstrates the potential utility of serum autoantibodies as diagnostic targets for RA subgroups and reveals potential therapeutic treatments.
To cite this abstract in AMA style:
Cunningham K, Hur B, Davis J, Sung J. Patients with ACPA-positive and ACPA-negative Rheumatoid Arthritis Show Different Circulating Auto-antibody Repertoires [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/patients-with-acpa-positive-and-acpa-negative-rheumatoid-arthritis-show-different-circulating-auto-antibody-repertoires/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patients-with-acpa-positive-and-acpa-negative-rheumatoid-arthritis-show-different-circulating-auto-antibody-repertoires/