Session Information
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: The risk of developing cardiovascular disease (CVD) through atherosclerosis in juvenile-onset systemic lupus erythematosus (JSLE) patients is significantly increased. This study aimed to stratify and characterize JSLE patients at elevated CVD-risk using patient/disease-related factors and metabolomic data from patients recruited to the APPLE (Atherosclerosis Prevention in Paediatric Lupus Erythematosus) clinical trial, designed to assess atherosclerosis development.
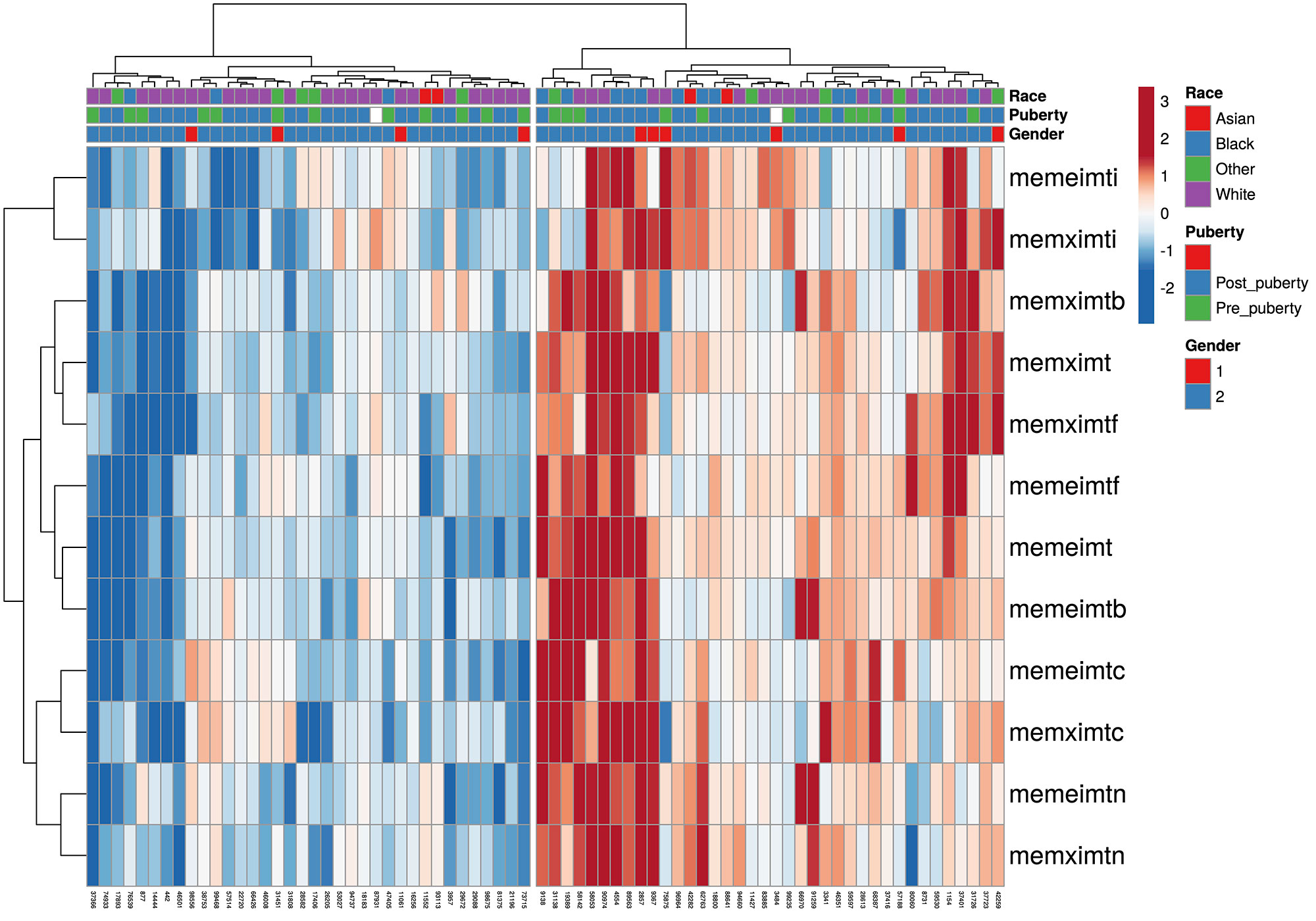
Methods: Unsupervised hierarchical clustering was performed to stratify patients by carotid intima-media thickness (cIMT) measurements at baseline (N=151) and cIMT progression over 36 months (placebo arm only, N=60). Baseline metabolomic profiles (250 serum metabolites) were compared between clusters using conventional statistics, univariate logistic regression, sparse Partial Least-Squares Discriminant Analysis and random forest classifier. An independent cohort (UCL-JSLE cohort, N=89) with matching metabolomics, immunophenotyping and proteomics, was used to validate the discovered CVD risk-related signatures from the APPLE cohort.
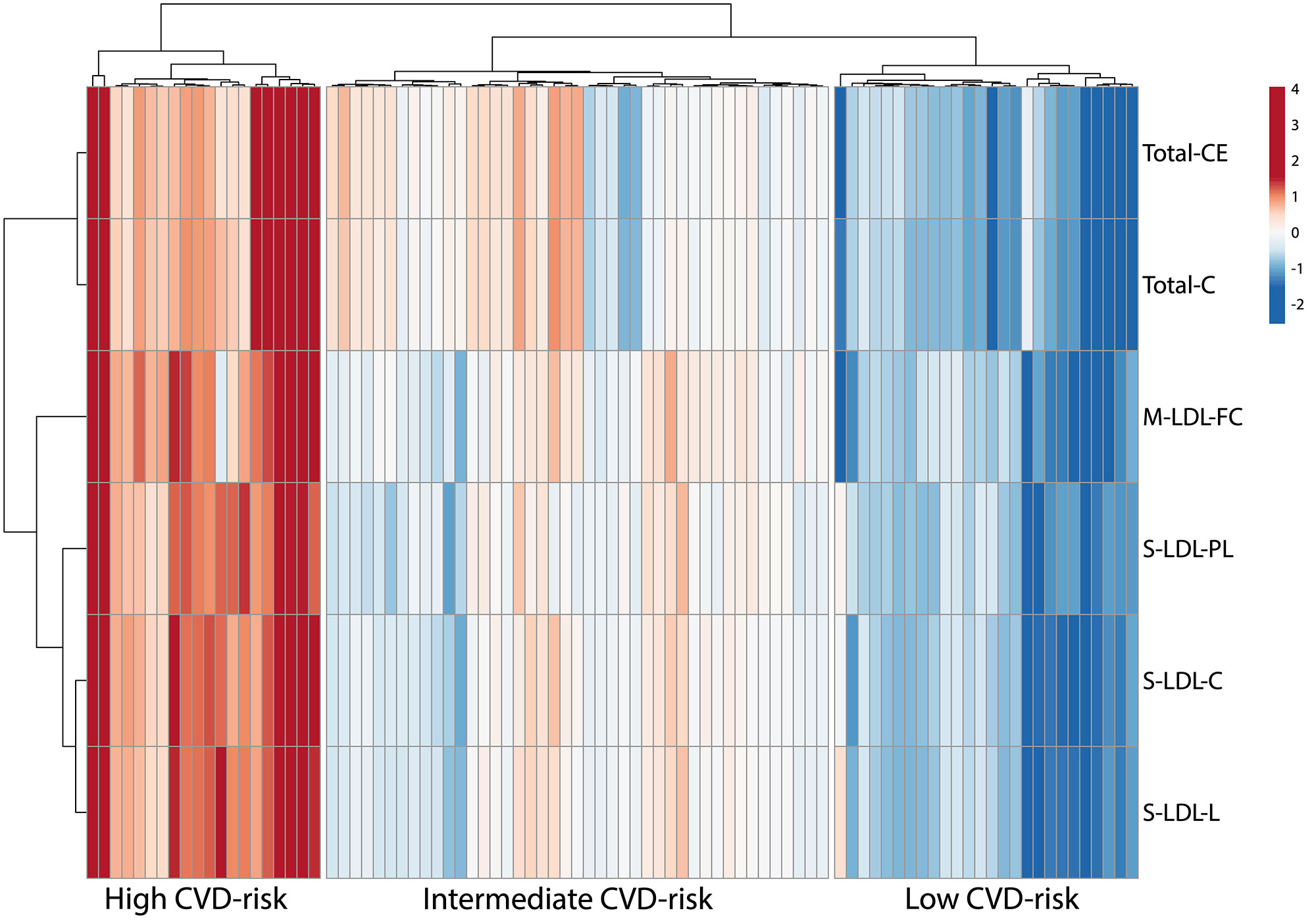
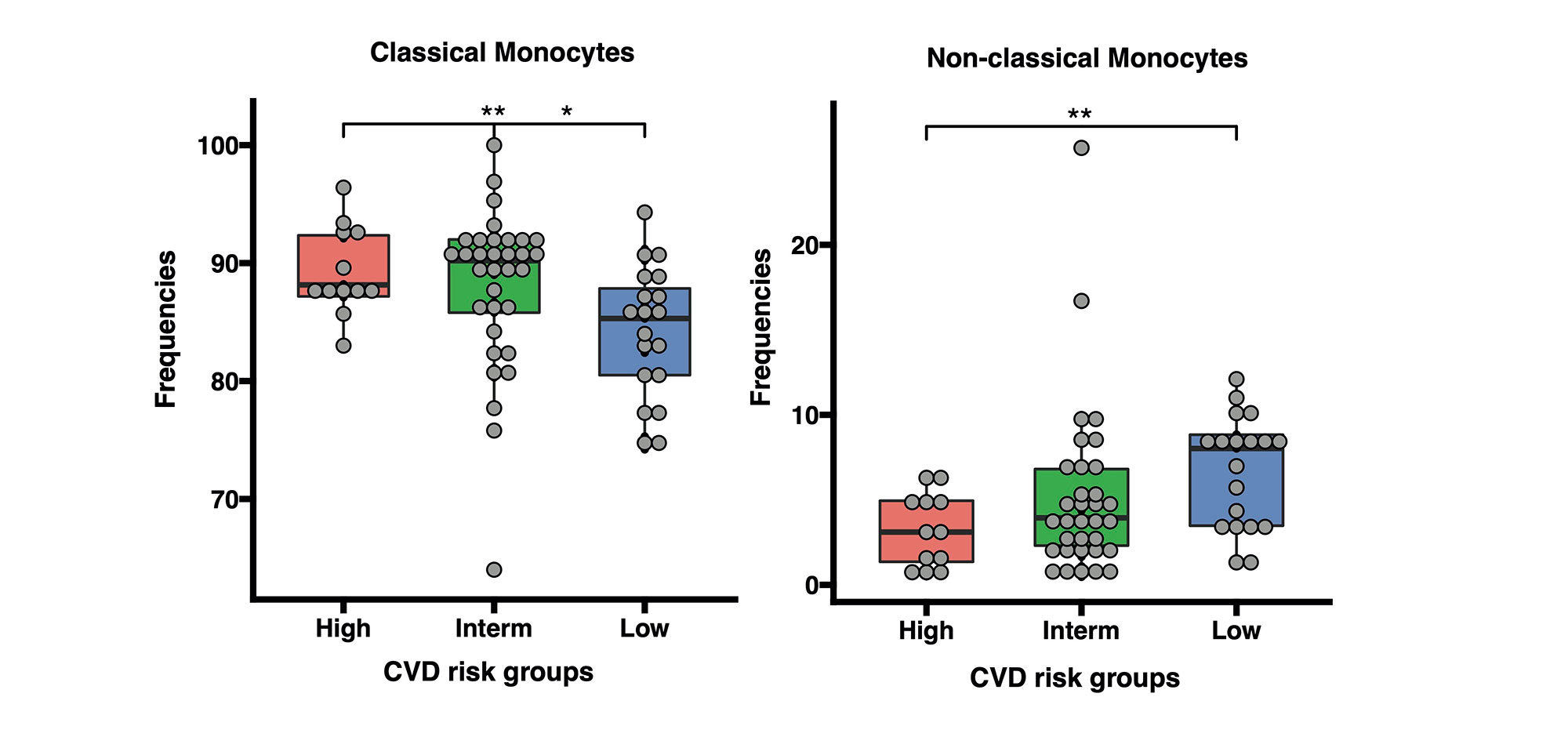
Results: Baseline cIMT stratification of all patients identified 3 clusters with high, intermediate, and low baseline cIMT measurements and progression trajectories over 36 months, each having distinct racial/BMI/household education/income characteristics. Analysis of cIMT progression over 36 months (ΔcIMT) in the placebo group only identified 2 patient groups with high and low cIMT progression. Unique metabolomic profiles differentiated high and low cIMT progression groups, with good discriminatory ability (0.81 AUC in ROC analysis) using the top 6 metabolites (Total cholesterol esters, Total cholesterol, Phospholipids in small LDL particles, Total cholesterol in small LDL particles, Free cholesterol in medium LDL particles and Total lipids in small LDL particles) selected from the analysis. ΔcIMT in the placebo group also correlated positively with baseline disease activity (SLEDAI), damage score (SLICC), white blood cell count, serum complement C3, blood pressure (both systolic and diastolic) and BMI. Metabolomics signatures discovered from the APPLE placebo cohort were applied to stratify JSLE patients in the validation cohort (UCL-JSLE), where 3 groups were identified with distinct metabolomics profiles indicating JSLE patients with potentially high (N= 20), intermediate (N= 43) and low (N= 26) CVD-risk according to metabolomic stratification. Significant differences were observed in the frequency of classical monocytes (p=0.015) and nonclassical monocytes (p=0.005) when comparing high and low CVD-risk group in the UCL-JSLE cohort.
Conclusion: Complex analysis of cIMT patterns and progression in the APPLE trial cohort identified novel key determinants that could guide further research for CVD-risk stratification in JSLE.
To cite this abstract in AMA style:
Peng J, Robinson G, Ardoin S, Schanberg L, Jury E, Ciurtin C. Patient-specific and Disease-related Determinants for Cardiovascular Disease (CVD) Risk Stratification in the APPLE (Atherosclerosis Prevention in Paediatric Lupus Erythematosus) Clinical Trial Cohort [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/patient-specific-and-disease-related-determinants-for-cardiovascular-disease-cvd-risk-stratification-in-the-apple-atherosclerosis-prevention-in-paediatric-lupus-erythematosus-clinical-trial-cohort/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-specific-and-disease-related-determinants-for-cardiovascular-disease-cvd-risk-stratification-in-the-apple-atherosclerosis-prevention-in-paediatric-lupus-erythematosus-clinical-trial-cohort/