Session Information
Date: Monday, October 27, 2025
Title: (1248–1271) Patient Outcomes, Preferences, & Attitudes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patient Reported Outcomes (PROs) collected via a mobile health application (App) can provide valuable insight into the health of patients with Rheumatoid Arthritis (RA). When collected at frequent intervals, providers can use them to assist in making decisions involving treatment intensification. We conducted a study to determine whether a single PRO score and/or sustained worsening in PROs were associated with future treatment intensification.
Methods: 150 patients with RA at one academic medical center were prompted to answer 1 of 4 validated PROs every other day through a mobile App (“mPRO”) for 1 year; thus, each of the PROs were answered in an 8-day cycle. The PROs included the RA Disease Activity Index 5 (RADAI-5) and PROMIS short forms of pain interference, fatigue, and physical function. Lower function scores are better, where higher scores are worse for the other PROs.The outcome of interest was RA treatment intensification – an increase in dose, frequency, or new use of any DMARD, NSAID, opioid, or oral glucocorticoid – during the App use period compared to the year prior. We examined 2 aspects of the PRO scores as potential predictors: the short-term PRO score during a given cycle and sustained PRO worsening defined as a clinically significant change compared to the previous measurement that was sustained for 3+ PRO cycles. Treatment intensification at the cycle immediately on or following each PRO score and sustained worsening was assessed. We used latent class mixed models to visualize trajectories of each PRO. Then, we examined mixed-effect logistic regression models to estimate the association between the time-varying PROs and treatment intensification. We conducted sensitivity analysis on a secondary outcome that excluded NSAIDs and opioids.
Results: Of the 150 patients who used the App, 83% were female, 91% were white, 7% had RA for less than 2 years, and the median age was 62 yrs. Over the study period, 284 treatment intensifications occurred among 63 patients, with a mean 1.9 (SD 3.2) changes per participant (Figure 1). Figure 2 depicts the trajectories of each PRO. The percentage with each trajectory differed by PRO: worsening — 23% for pain, 18% for fatigue, 26% for function, 10% for RADAI; improvement — 14% for pain, 12% for fatigue, 8% for function, 9% for RADAI; and stable –63% for pain, 70% for fatigue, 66% for function, 80% for RADAI.Regression models showed an association with treatment intensification and both short-term PROs and sustained worsening of the PROs. The intensifications were more likely to occur after a sustained worsening of pain interference (OR 2.04, 95% CI 1.10-3.78) or fatigue (OR 2.01, 95% CI 1.13-3.57). All four multivariable models (one for each PRO) had AUCs > 0.73, indicating moderate to strong discrimination. Models with the secondary outcome (not NSAIDs or opioids) showed similar results, with some stronger odds ratios but larger confidence intervals (Table 1).
Conclusion: Our results show that longitudinally measured PROs can identify periods of worsening pain and fatigue, which are correlated with subsequent treatment intensification. Frequently assessed PROs can help augment clinical decision-making by providing symptom information between visits.
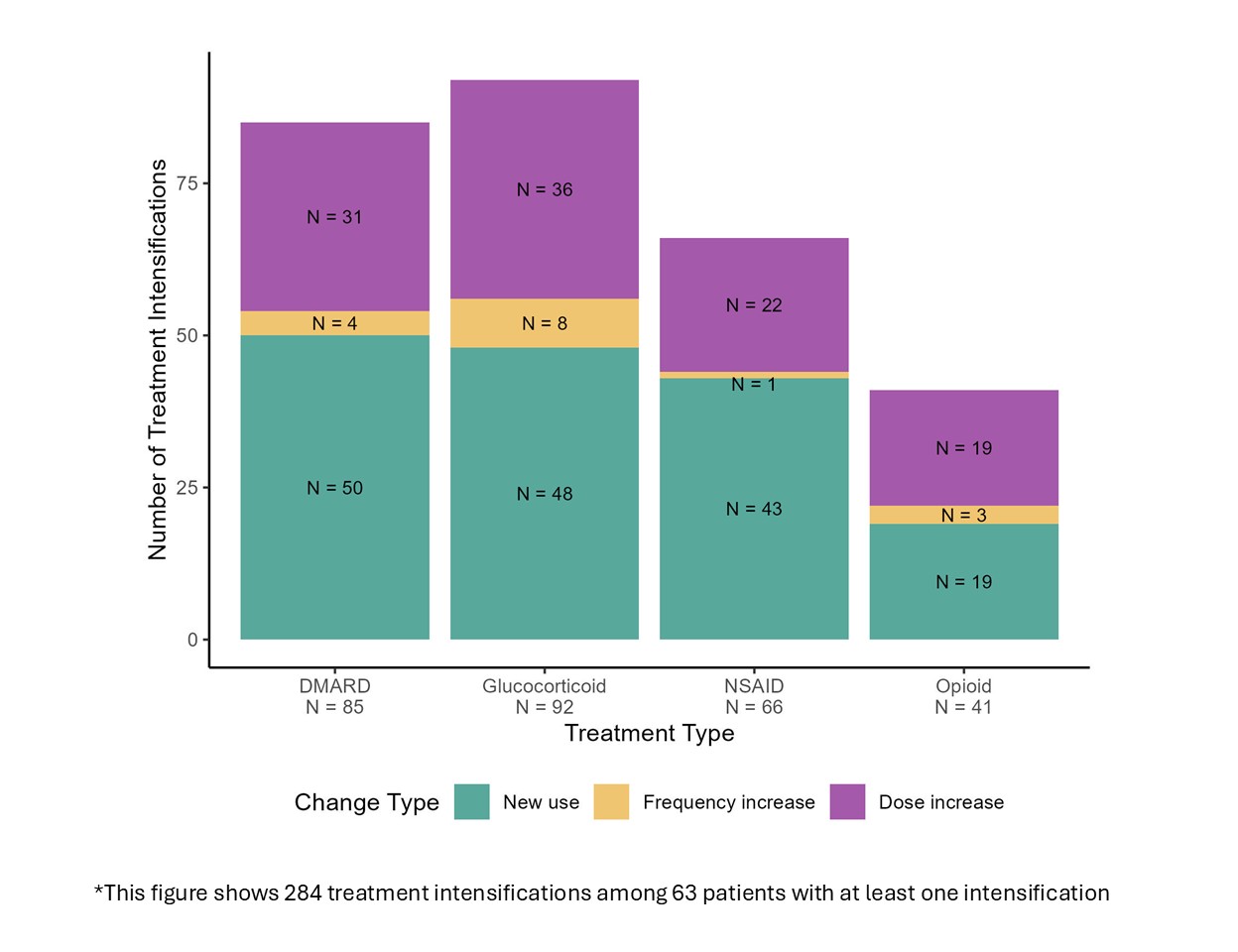 Figure 1. Types of treatment intensifications that occurred during a one-year study period among RA patients with at least one intensification.
Figure 1. Types of treatment intensifications that occurred during a one-year study period among RA patients with at least one intensification.
.jpg) Figure 2. Predicted trajectories of pain interference, fatigue, physical function, and RADAI over one year among patients with RA.
Figure 2. Predicted trajectories of pain interference, fatigue, physical function, and RADAI over one year among patients with RA.
.jpg) Table 1. The Impact of PRO changes on treatment intensification among patients with RA
Table 1. The Impact of PRO changes on treatment intensification among patients with RA
To cite this abstract in AMA style:
Santacroce L, Paudel M, Solomon D. Patient Reported Outcomes Predict Subsequent Treatment Intensification Among Rheumatoid Arthritis Patients: Longitudinal PRO Measurement Using a Mobile Health App [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/patient-reported-outcomes-predict-subsequent-treatment-intensification-among-rheumatoid-arthritis-patients-longitudinal-pro-measurement-using-a-mobile-health-app/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-predict-subsequent-treatment-intensification-among-rheumatoid-arthritis-patients-longitudinal-pro-measurement-using-a-mobile-health-app/
