Session Information
Date: Monday, November 9, 2020
Title: RA – Diagnosis, Manifestations, & Outcomes Poster IV: Lifespan of a Disease
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients with active rheumatoid arthritis (RA), treatment with upadacitinib (UPA) has resulted in clinically meaningful improvements in patient-reported outcomes (PROs). This post hoc analysis of SELECT-CHOICE (NCT03086343), a phase 3, double-blind randomized clinical trial, evaluated the benefits of UPA vs intravenous (IV) abatacept (ABA) with background conventional synthetic DMARDs (csDMARDs) on PROs in patients with active RA and inadequate response or intolerance to biologic DMARDs (bDMARD-IR).
Methods: Patients in SELECT-CHOICE received UPA (oral 15 mg once daily) or ABA (IV) while on stable csDMARD therapy. PROs included: Patient Global Assessment of Disease Activity (PtGA) by visual analog scale (VAS), patient’s assessment of pain by VAS, Health Assessment Questionnaire Disability Index (HAQ-DI), duration and severity of morning (AM) stiffness, 36-Item Short Form Health Survey (SF-36), Functional Assessment of Chronic Illness Therapy-Fatigue, Work Productivity and Activity Impairment (WPAI), and EuroQol 5-dimension 5-level (EQ-5D-5L) index score. Least squares mean (LSM) changes from baseline (BL) to Week 12 were based on an analysis of covariance model. The proportions of patients reporting improvements from BL to Week 12 that were ≥ minimal clinically important differences (MCID) or scores ≥ normative values were calculated for UPA and ABA treatment; comparisons used chi-square tests. Time to response, defined as improvement ≥ MCID, was assessed by Kaplan-Meier analysis and compared using the log-rank test.
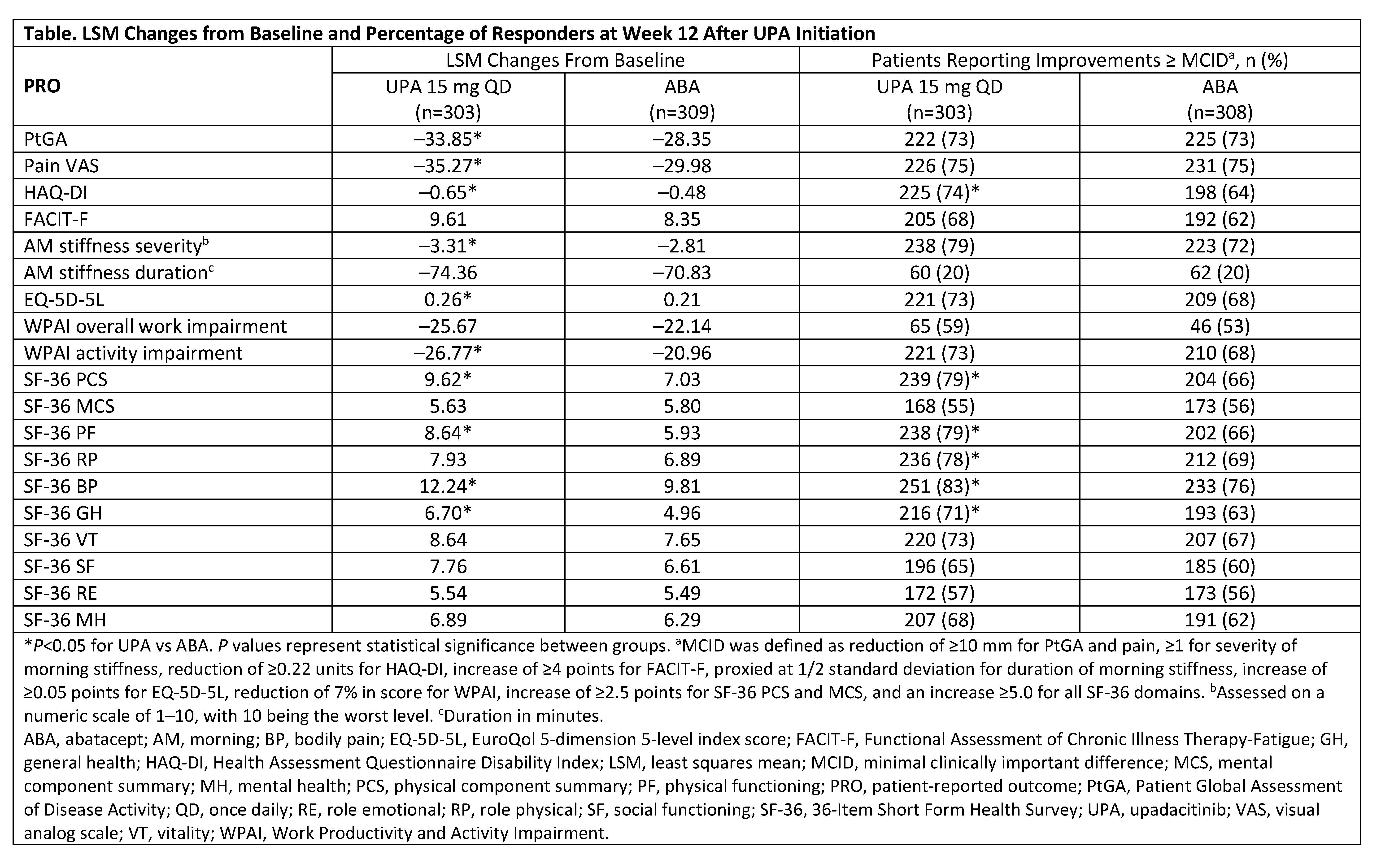
Results: Data from 612 patients were analyzed (303 received UPA and 309 received ABA). The mean age was 56 years and mean duration of RA was 12 years. Thirty-two percent received >1 prior bDMARD and 72% received concomitant MTX at BL. At Week 12, UPA treatment resulted in statistically significant LSM changes from BL vs ABA in PtGA, pain, HAQ-DI, severity of AM stiffness, EQ-5D-5L, WPAI activity impairment domain, and in 3/8 SF-36 domains and physical component summary (PCS) score (Table). Compared with ABA at Week 12, significantly more UPA-treated patients reported improvements ≥ MCID in HAQ-DI and in 4/8 SF-36 domain scores and PCS score. The proportion of UPA- vs ABA-treated patients reporting scores ≥ normative values were significantly greater in PtGA (37% vs 23%), HAQ-DI (18% vs 10%), EQ-5D-5L (22% vs 13%), and SF-36 PCS (17% vs 8%), including physical functioning (21% vs 11%), bodily pain (33% vs 23%), and general health (24% vs 17%) domains. The median time to response was significantly shorter (P< 0.01) for UPA- vs ABA-treated patients in HAQ-DI (2 weeks vs 4 weeks).
Conclusion: Among bDMARD-IR patients with moderate to severely active RA, treatment with UPA or ABA resulted in clinically meaningful improvements in PROs at Week 12. Overall, greater improvements in PROs especially in the key domains of physical functioning, pain, and general health, were observed with treatment with UPA compared with ABA; improvements in HAQ-DI were observed earlier in UPA- vs ABA-treated patients.
Medical writing services provided by Brandy Menges of JK Associates, Inc. (a member of Fishawack Group of Companies; Conshohocken, PA) and funded by AbbVie.
To cite this abstract in AMA style:
Bergman M, Enejosa J, Martin N, Suboticki J, Goldschmidt D, Song Y, Tundia N. Patient-Reported Outcomes of Upadacitinib versus Abatacept in Patients with Rheumatoid Arthritis and an Inadequate Response to Biologic Disease-Modifying Antirheumatic Drugs: 12-Week Results of a Phase 3 Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/patient-reported-outcomes-of-upadacitinib-versus-abatacept-in-patients-with-rheumatoid-arthritis-and-an-inadequate-response-to-biologic-disease-modifying-antirheumatic-drugs-12-week-results-of-a-phas/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-of-upadacitinib-versus-abatacept-in-patients-with-rheumatoid-arthritis-and-an-inadequate-response-to-biologic-disease-modifying-antirheumatic-drugs-12-week-results-of-a-phas/