Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster I: RA, Spondyloarthritis, & OA
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
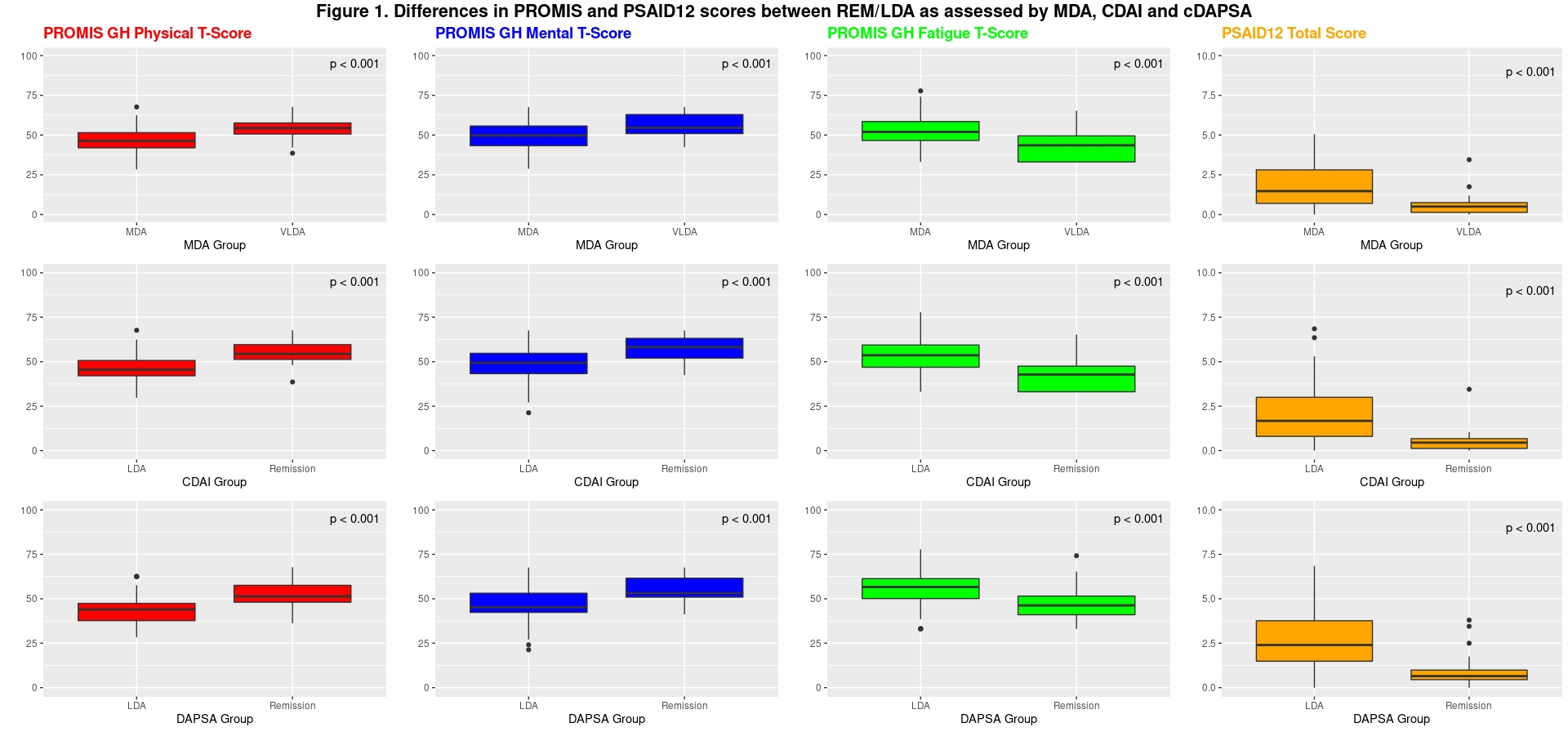
Background/Purpose: For psoriatic arthritis (PsA), several different composite instruments are available to define low disease activity (LDA) and remission (REM) targets for treatment. Patient-reported outcomes (PROs) may also be useful in assessing disease activity and may be more practical than composite indices in some settings. In this study, we examined the ability of PROs to differentiate between states of low disease activity and remission treatment targets (LDA and REM), using composite indices as the reference standards.
Methods: This cross-sectional study was performed with the Psoriatic Arthritis Research Consortium between 2016-2019. PROs included Patient-Reported Outcomes Measurement Information System [PROMIS] instruments, EULAR Psoriatic Arthritis Impact of Disease [PSAID12], and Routine Assessment of Patient Index Data 3 [RAPID3]). Participants (pts) were classified as LDA if they fulfilled composite index criteria for Minimal Disease Activity (MDA), Clinical Disease Activity Index (CDAI)-LDA, or Disease Activity in Psoriatic Arthritis (cDAPSA)-LDA and REM if they fulfilled composite index criteria for Very Low Disease Activity (VLDA), CDAI-REM, or cDAPSA-REM. PROs were evaluated by determining 1) score differences between pts in LDA vs. REM, 2) correlations with composite indices scores, and 3) percentages of pts in LDA and REM who fulfilled PRO criteria for low disease states (in PROs with previously established low disease state criteria). PROs were compared between groups using t-tests or Wilcoxon rank sum test, depending on their distributions. The categorical versions of RAPID3 and PSAID12 were compared between groups using Chi-Squared test or Fisher’s exact test, when appropriate. Correlations were calculated with Spearman’s rank correlation. Data was analyzed using R software (Version 3.5; Vienna, Austria).
Results: 227 PsA pts were included (52.2% female, average age 52.7±14 years). Compared to pts in LDA, pts in REM had significantly more favorable PROMIS Physical, PROMIS Mental, PROMIS Fatigue, and PSAID12 scores (Figure 1). Correlations were strong between the composite indices and PROMIS GH physical health (r=0.65- 0.69) and between the composite indices and PSAID12 (r=-0.77- 0.79) (Figure 2). RAPID3 Low Severity and Near-Remission were reported by >98% of patients in REM, but only up to 54% of patients in LDA (Table 1). PSAID12 Patient Acceptable State occurred more frequently in pts in DAPSA-REM than pts in DAPSA-LDA (Table 1).
Conclusion: PROMIS and PSAID12 instruments correlated well with composite indices and differentiated between states of LDA vs REM. RAPID3 Near-Remission may be the most rigorous PRO criteria (including only the lowest states of disease activity), while PSAID Patient Acceptable State may identify a broader range of low states of disease activity. These data contribute to the construct validity of using PROs to measure low states of disease activity that may be considered for additional treatment targets in PsA.
To cite this abstract in AMA style:
Yedimenko J, Walsh J, Ogdie A, Jin Y, Reddy S, Scher J, Husni M. Patient-Reported Outcomes Differentiate Between Remission and Low Disease Activity in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/patient-reported-outcomes-differentiate-between-remission-and-low-disease-activity-in-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-reported-outcomes-differentiate-between-remission-and-low-disease-activity-in-psoriatic-arthritis/