Session Information
Date: Monday, November 13, 2023
Title: (1200–1220) Patient Outcomes, Preferences, & Attitudes Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Brain fog is commonly reported by SJD patients, though limited evidence exists to understand patient experiences. Various terms may be used to describe brain fog, including mental or cognitive fatigue and cognitive dysfunction. While cognitive difficulties are typically assessed via battery testing, there is no brain fog PRO to assess related patient symptoms and impacts experienced by patients. The purpose of this study was to explore concepts related to brain fog among SJD patients, and attempt to discern whether brain fog is related to fatigue or may be a separate manifestation as experienced by SJD patients.
Methods: A cross-sectional qualitative study among adults with clinician-confirmed SJD consisted of 30-minute semi-structured concept elicitation (CE) interviews and a pile-sort exercise. A thematic approach, a codebook, and MAXQDA software were used to organize, code, and analyze anonymized CE interview transcripts. In the sorting exercise, patients ranked all items from the Neuro-QoL-Cognitive Function and PROMIS-Cognitive Fatigue item banks as most, somewhat, or not relevant to their brain fog experience. Cultural consensus analysis (CCA) was used to determine whether there were items that participants agreed were most relevant (consensus) that may be considered for a de novo PRO instrument.
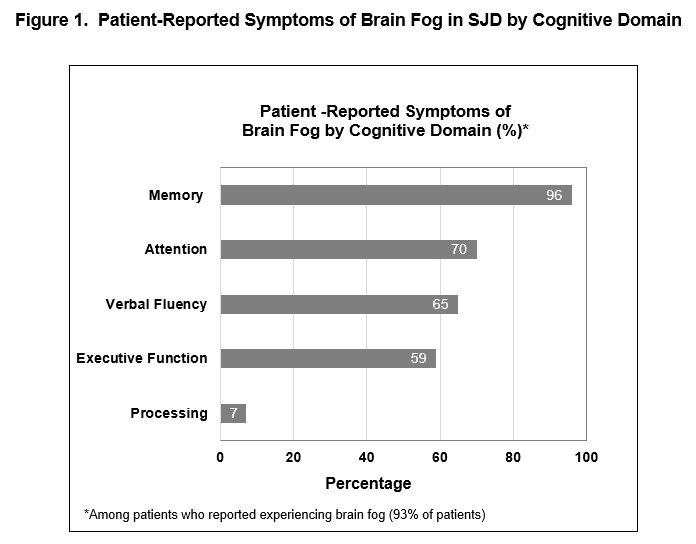
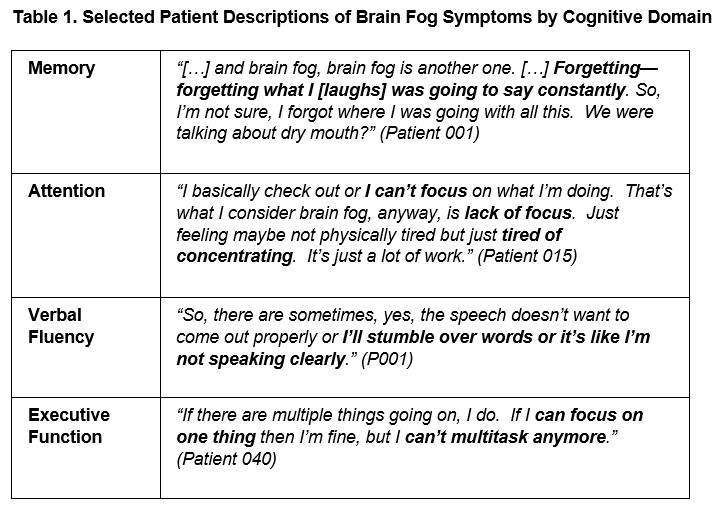
Results: Of N=29 SJD patients interviewed (mean age, 63 yrs; 97% female; 79% Caucasian; mean length of time since diagnosis, 13.6 yrs), 75% spontaneously used the term “brain fog” to describe their cognitive difficulties. Symptoms included deficits in memory (96%), concentration (70%), verbalizing (65%), and executive function (59%) (Figure 1, Table 1). Patients reported experiencing brain fog all the time (12%), daily (24%), weekly (36%), monthly (20%) or annually (8%). Results indicated patients generally do not associate brain fog directly with fatigue; of 11 (44%) patients describing brain fog and fatigue as somewhat related, 9 (82%) stated brain fog and fatigue can occur separately while 5 felt fatigue was triggering or causing their brain fog (45%). CCA results from the pile sort exercise indicated that consensus, defined as an Eigenvalue ratio of >3.1, existed among a participant subset (n=10; ER 4.05). Comparing results based on this subset and all patients demonstrated a nearly identical overlap in highly ranked items between the two groups. Neuro-QoL items consistently ranked higher than PROMIS items; 17 of the top 20 highest ranked items were based on the Neuro-QoL, indicating items of cognitive dysfunction were more relevant than those of cognitive fatigue for SJD patients.
Conclusion: Brain fog commonly affects SJD patients with symptoms impacting memory, concentration, verbalization and executive function. Participant endorsement of items from the Neuro-QoL suggests brain fog may be attributable to independent cognitive dysfunction rather than cognitive fatigue. The results support exploring the use of highly ranked Neuro-QoL items to develop a draft de-novo Brain Fog PRO for further research in SJD patients.
To cite this abstract in AMA style:
Kruzikas D, Eldred A, Kafka S, Church J, Hammitt K, Koochaki P, O'Donnell C. Patient Experience of Brain Fog to Inform the Development of a De Novo Patient Reported Outcome (PRO) in Patients with Sjögren’s Disease [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/patient-experience-of-brain-fog-to-inform-the-development-of-a-de-novo-patient-reported-outcome-pro-in-patients-with-sjogrens-disease/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-experience-of-brain-fog-to-inform-the-development-of-a-de-novo-patient-reported-outcome-pro-in-patients-with-sjogrens-disease/