Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The United States (US) Food and Drug Administration approved avacopan to treat adults with severe active granulomatosis with polyangiitis or microscopic polyangiitis in October 2021. Real-world data on patients receiving avacopan are limited. The current study evaluated demographics, clinical characteristics, and treatment patterns among individuals who initiated avacopan in the US.
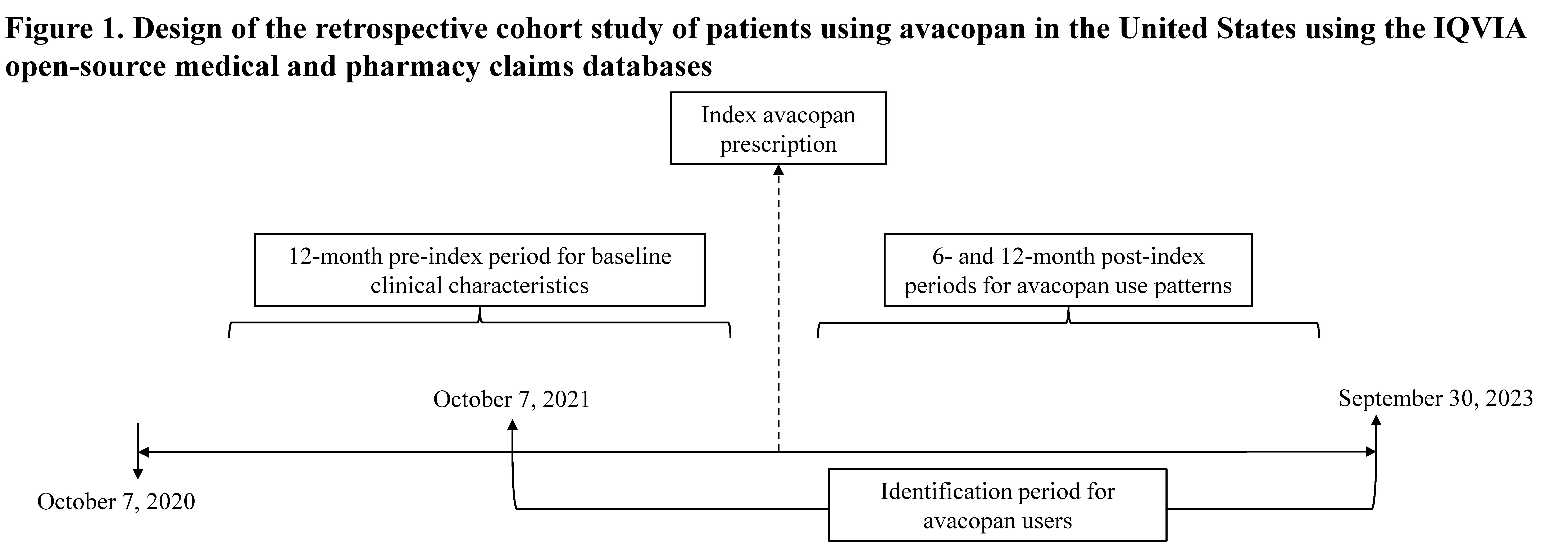
Methods: A retrospective cohort study was conducted using the IQVIA open-source medical claims (Dx) and longitudinal prescription (LRx) claims databases in the US. Dx includes professional and medical claims sourced through practice management systems and clearinghouses, while LRx represents claims sourced from the pharmacy point-of-sale. Patients with ≥1 avacopan claim from October 2021 to September 2023 were identified, with the first avacopan claim date being the index-date (Figure 1). Patients ≥18 years old with ≥12 months of pre-index continuous data (assessed using a proxy measure for continuous enrollment for open-source data) were included. Demographics at the index-date and clinical characteristics in the 12-month pre-index period were assessed. Avacopan use patterns were also evaluated among patients who met the proxy-based continuous enrollment criterion in the 6- and 12-month post-index periods, respectively.
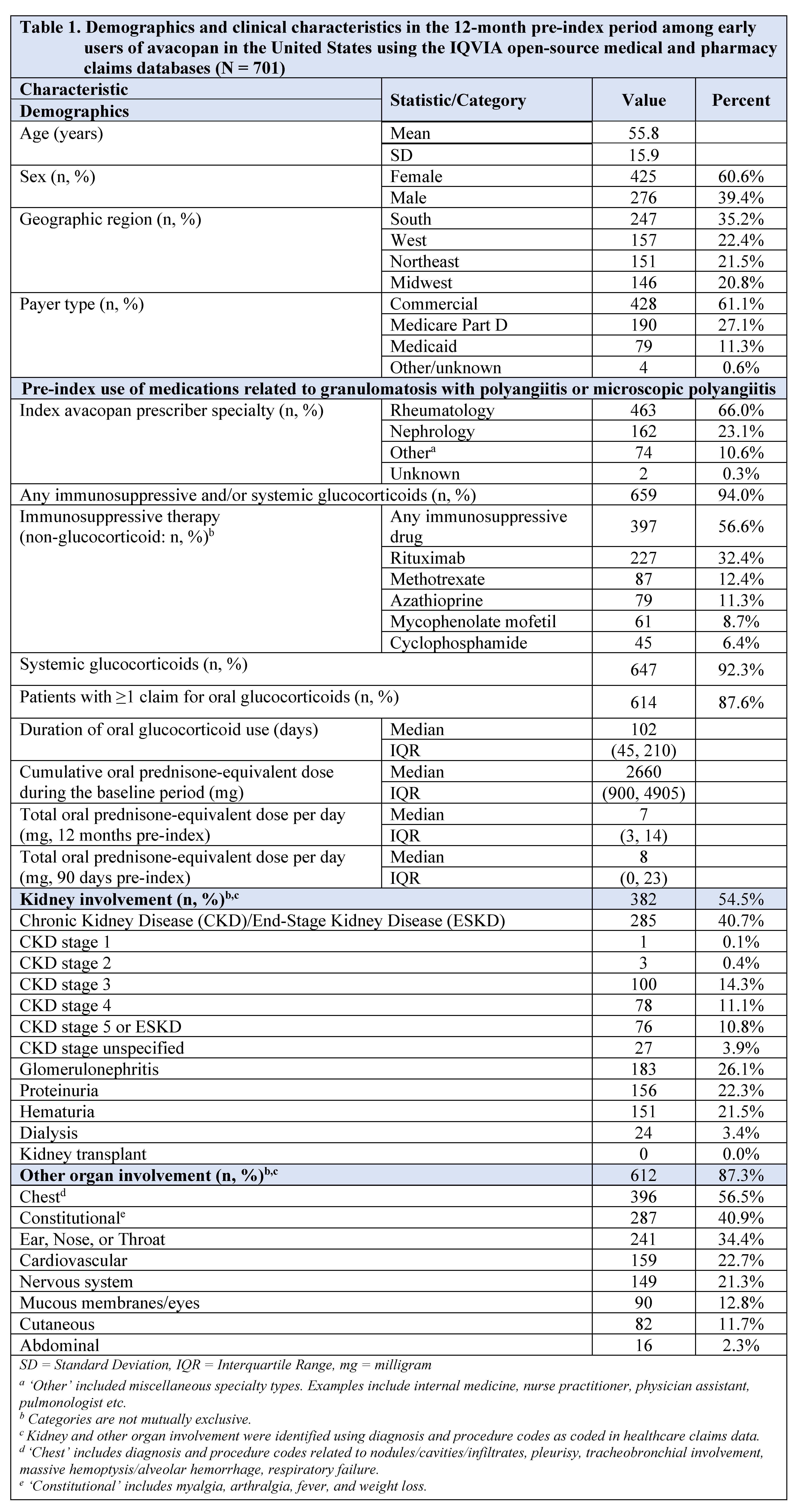
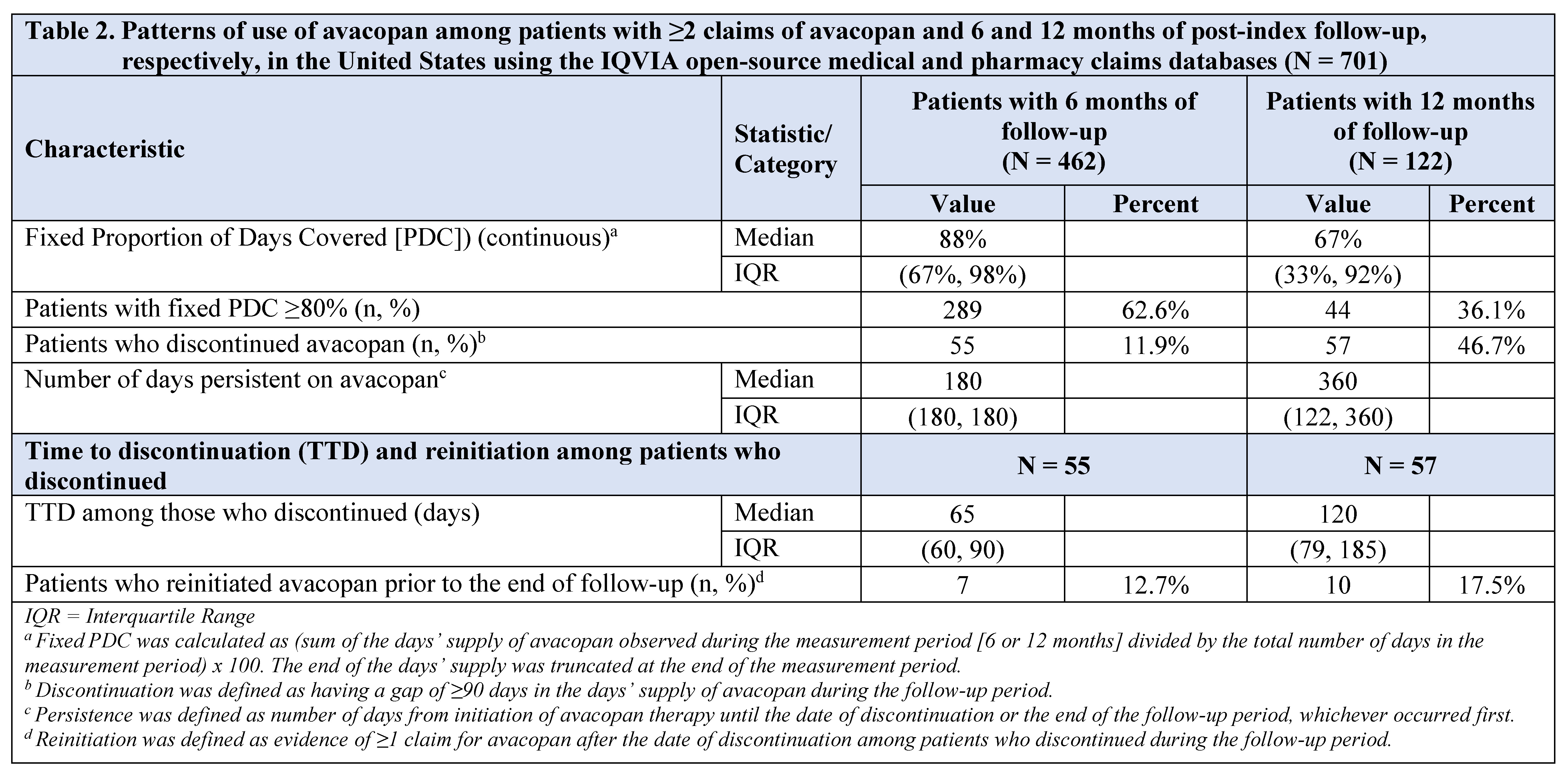
Results: Overall, 701 patients with ≥1 avacopan claim were identified: mean [SD] age: 55.8 [15.9] years, 60.6% female, 61.1% commercially insured (Table 1). Index avacopan prescription was mostly prescribed by rheumatologists (66.0%) or nephrologists (23.1%). Before starting avacopan, 92.3% and 56.6% received systemic glucocorticoids (GCs) and other immunosuppressive therapy, respectively. In the pre-index period, pulmonary (56.5%), kidney (54.5%), constitutional (40.9%), and ear, nose, or throat (34.4%) involvement were commonly observed. The median duration of follow-up among patients was 294 (IQR: 215-341) days. A total of 462 (65.9%) and 122 (17.4%) patients had ≥2 avacopan claims and at least 6 and 12 months of follow-up, respectively (Table 2). Of the 462 patients with ≥6 months of follow-up, 55 (11.9%) discontinued avacopan within 6 months, and the median time from start to discontinuation among those who discontinued was 65 (IQR: 60-90) days. Of the 122 patients with ≥12 months of follow-up, 57 (46.7%) discontinued avacopan within 12 months, and the median time from start to discontinuation among those who discontinued was 120 (IQR: 79-185) days. There were 7 (of 55) and 10 (of 57) patients who reinitiated avacopan in the 6- and 12-month cohorts, respectively.
Conclusion: Among initial users of avacopan in the US, most had kidney and pulmonary involvement and used GCs in the year before starting avacopan. Most patients with 6 and 12 months of follow-up continued avacopan use over the respective periods. Future studies will assess outcomes following treatment with avacopan in a real-world setting.
To cite this abstract in AMA style:
Inguva S, Rane P, Trimpe D, Oh S, Multani J, Chang H, Yasuda M, Chen C, Geetha D, Merkel P, Wallace Z. Patient Characteristics and Treatment Patterns Before and After Initiation of Avacopan in the United States: An Early View Based on a Claims Database Analysis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/patient-characteristics-and-treatment-patterns-before-and-after-initiation-of-avacopan-in-the-united-states-an-early-view-based-on-a-claims-database-analysis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/patient-characteristics-and-treatment-patterns-before-and-after-initiation-of-avacopan-in-the-united-states-an-early-view-based-on-a-claims-database-analysis/