Session Information
Date: Saturday, November 16, 2024
Title: SLE – Animal Models Poster
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Lupus nephritis is the most common life-threatening end-organ complication of SLE, and response rates to current interventions remain poor. Notably, PD-L1 and other interferon-responsive genes are upregulated in the kidneys of both humans and mice with lupus nephritis. We and others have shown that the kidney parenchyma has immunosuppressive effects on infiltrating immune cells. We hypothesize that the kidney parenchyma upregulates several factors in response to inflammatory signals, which can then dampen inflammatory signals to reduce local damage in the kidney. As IFNγ is the major inducer of PD-L1, we postulated that IFNγ induces this protective program in the kidneys of lupus-prone mice.
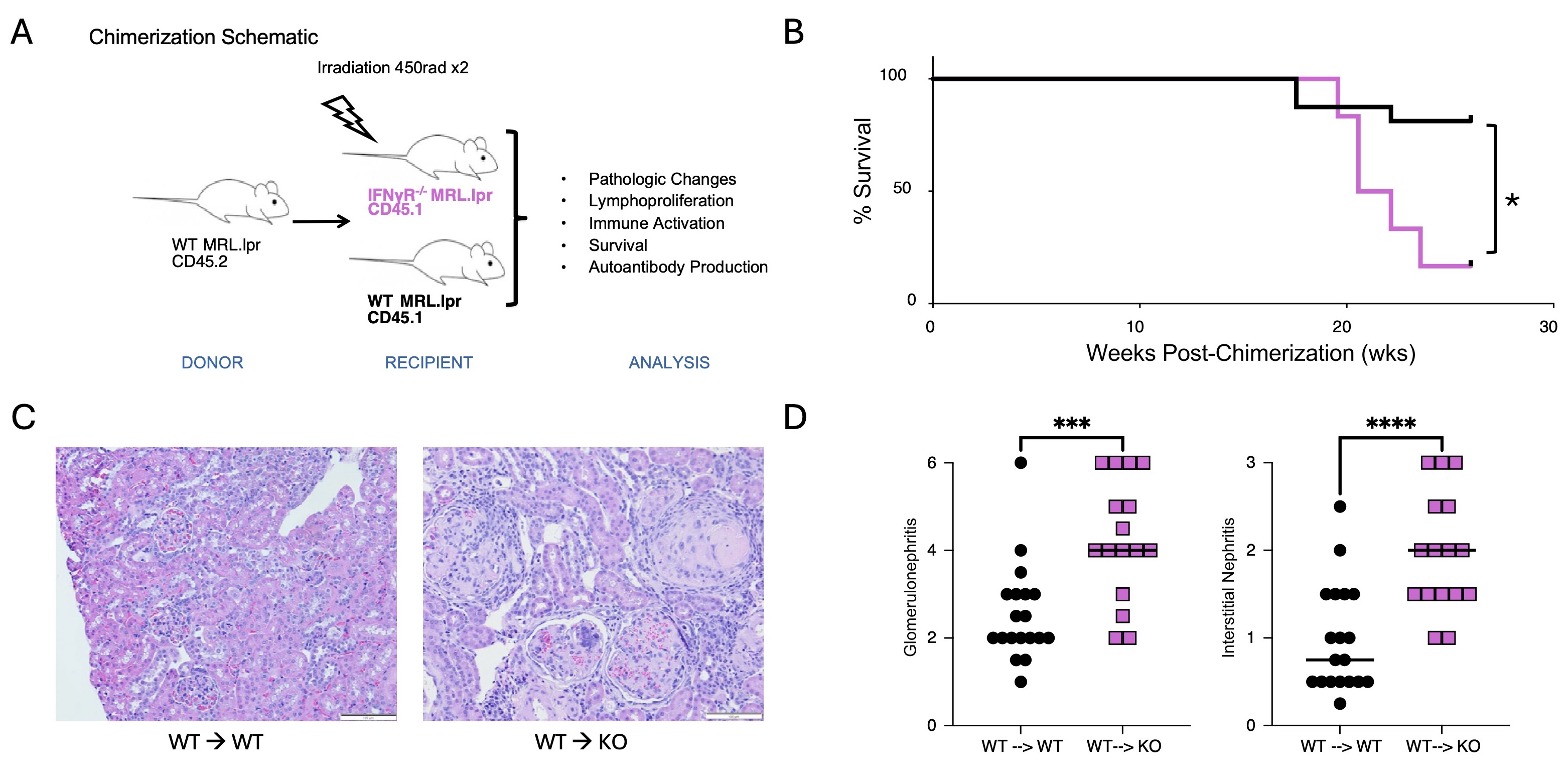
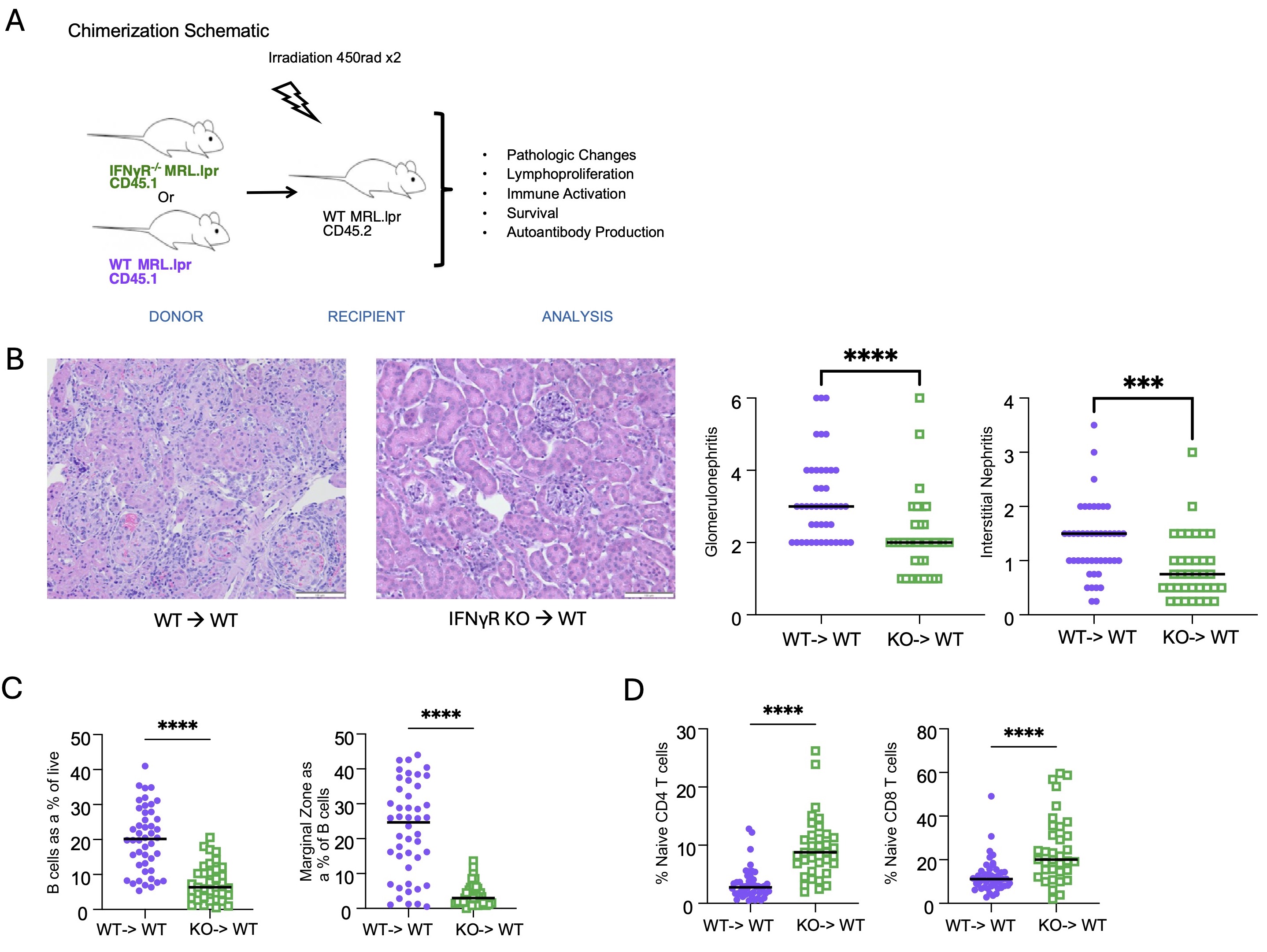
Methods: MRL.Faslpr mice develop autoantibodies, proteinuria, dermatitis, and glomerulonephritis. In this study, we created parenchymal bone marrow chimeras in which congenically labeled wild-type (WT) immune cells are transferred into either WT MRL.Faslpr, IFNγR-/- MRL.Faslpr, or PD-L1-/- MRL.Faslpr recipients (Fig 1A). Based on our hypothesis, we predicted that both the IFNγR-/- and PD-L1-/- recipients would have more severe disease than WT controls. We also performed reverse chimeras in which WT or IFNγR-/- bone marrow was transferred into WT MRL.Faslpr recipients to assess the role of IFN signaling in the hematopoietic compartment (Fig 2A). Analysis included measurement of proteinuria, histological assessment of interstitial and glomerular disease, autoantibody quantification, FACS analysis of immune cell activation, and survival analysis.
Results: Consistent with our hypothesis, IFNγR-/- MRL.Faslpr recipient mice demonstrated more severe and rapid disease onset than WT recipient controls; this included higher rates of mortality (p< 0.05), more severe glomerulonephritis (p< 0.005), and interstitial disease (p< 0.001) (Fig 1). While parenchymal IFNγR deficiency resulted in reduced PD-L1 expression, unexpectedly, the PD-L1-/- MRL.Faslpr recipient mice did not exhibit an exacerbated phenotype, suggesting an alternative regulatory mechanism induced by IFNγ in the kidney of lupus-prone mice. Conversely, when IFNγR was deleted in the hematopoietic compartment, mice exhibited ameliorated disease (Fig 2).
Conclusion: Immune signaling mediated by IFNγ has dichotomous effects in lupus-prone mice. In the parenchyma, IFNγ regulates an anti-inflammatory pathway that surprisingly is independent of PD-L1, while in the hematopoietic compartment, IFNγ has pro-inflammatory properties. These findings are key to informing future therapeutic development as inhibiting receptors or inflammatory signaling pathways on specific cell types may be more efficacious than targeting these pathways systemically. This may explain why anifrolumab, which is efficacious for systemic disease, has had suboptimal responses in lupus nephritis trials as it may lead to proinflammatory effects in the kidney. These findings may also aid our understanding of the fundamental biology of disease; for instance, they demonstrate that the kidney is not an innocent bystander, but can play a role in regulating inflammation, which could explain underlying tissue-specific disease heterogeneity.
To cite this abstract in AMA style:
Kim M, Marinov A, Cosgrove H, Gong X, Shlomchik M, Tilstra J. Parenchymal and Hematopoietic IFNγ Signaling Exhibits Dichotomous Effects in Murine Lupus [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/parenchymal-and-hematopoietic-ifn%ce%b3-signaling-exhibits-dichotomous-effects-in-murine-lupus/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/parenchymal-and-hematopoietic-ifn%ce%b3-signaling-exhibits-dichotomous-effects-in-murine-lupus/