Session Information
Date: Saturday, November 6, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Diagnosis (0323–0356)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry is a multi-center, observational registry that collects demographic, clinical, and provider- and patient-reported data from patients with pediatric-onset rheumatic diseases in North America, Israel and Italy. This study aimed to describe the demographic features, cumulative clinical manifestations, and treatments of the childhood systemic lupus erythematosus (cSLE) cohort within the CARRA Registry.
Methods: Since 2015, the CARRA Registry has enrolled 10,411 patients at 70 centers. Childhood-onset SLE enrollment began in March 2017. We performed a retrospective cohort study of patients with cSLE enrolled in the US between March 2017 to December 2020. Inclusion criteria for participants in the CARRA cSLE Registry include: 1) diagnosis of cSLE at < 18 years based on American College of Rheumatology (ACR) or Systemic Lupus Erythematosus International Collaborating Clinics (SLICC) criteria; 2) enrollment within two years of cSLE diagnosis or at the time of a flare of lupus nephritis; and 3) enrollment prior to 21 years of age. Sociodemographic and clinical data were summarized using descriptive statistics.
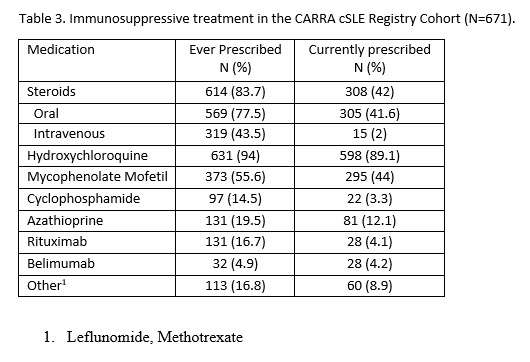
Results: The current registry cohort includes 671 participants with cSLE. The majority are female (85%) with mean age at enrollment of 14.3 (SD 2.9) years. The cohort is both ethnically and racially diverse (Table 1). Socioeconomic status varies widely, noting 12.5% having a household income below $25,000/year. The median time from symptom onset to diagnosis was two months (interquartile range (IQR) 25 days to 6 months), from diagnosis to enrollment was 5 (IQR 1-15) months, and from enrollment to end of follow up was 14 (IQR 6 to 23) months. At the end of the follow-up period, more than 60% of participants developed nephritis as defined by ACR or SLICC criteria. 6.1% and 10% had neurological manifestations per ACR and SLICC criteria, respectively (Table 2). Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) at enrollment was a median of 4 (IQR 2-10). Most patients were prescribed hydroxychloroquine. In the first 2-3 years of disease, participants received a variety of immunosuppressive therapies including Mycophenolate Mofetil, Cyclophosphamide, Azathioprine, Rituximab, Belimumab and disease modifying anti-rheumatic drugs such as Leflunomide and Methotrexate. 84% of patients were prescribed either oral or intravenous glucocorticoids during their disease course (Table 3).
Conclusion: The CARRA Registry has enrolled a racially and ethnically diverse cohort of cSLE patients in the early course of their disease. These participants exhibit moderate disease activity and although the use of hydroxychloroquine in this cohort is high, a significant proportion of patients are utilizing glucocorticoids at the last study visit. We anticipate enrolling a minimum of 1000 participants with more than ten years of follow-up. This cohort, which is one of the Centers for Disease Control (CDC) funded SLE registries, provides a unique opportunity to describe the natural history, treatments, and outcomes in patients with cSLE.
To cite this abstract in AMA style:
Bacha C, Dennos A, Knight A, Schanberg L, Son M, von Scheven E, Amin S, Helmick C, Hersh A. Overview of the Childhood Systemic Lupus Erythematosus (cSLE) Cohort in the CARRA Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/overview-of-the-childhood-systemic-lupus-erythematosus-csle-cohort-in-the-carra-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/overview-of-the-childhood-systemic-lupus-erythematosus-csle-cohort-in-the-carra-registry/