Session Information
Session Type: Abstract Session
Session Time: 3:30PM-3:45PM
Background/Purpose: Timely diagnosis of children, adolescents and young adults with rheumatologic disorders remains a global challenge especially in lower resource countries and areas (LRCs). There are several LRCs on the African continent with limited access to antinuclear antibodies (ANA) testing necessary to support a diagnosis of several pediatric rheumatic disorders (PRD; % of patients with positive ANA) including systemic lupus erythematosus (100%), juvenile dermatomyositis (60-75%), and juvenile idiopathic arthritis (40-60%). As the presence of ANA has near-ubiquitous utility in initially evaluating for a rheumatic condition, we aimed to understand physician perspectives on the barriers to ANA testing in pediatric populations across Africa, and the regional differences in barriers.
Methods: Electronic REDCap surveys were sent to physicians within the Pediatric Society of the African League Against Rheumatism (PAFLAR). Surveys were sent via individual email links from January to March 2025. Dillman’s methods for surveys were used to optimize survey response rates. Descriptive analysis was done and comparisons considering geographic region and the World Bank classification of economies based on Gross National Income per capita.
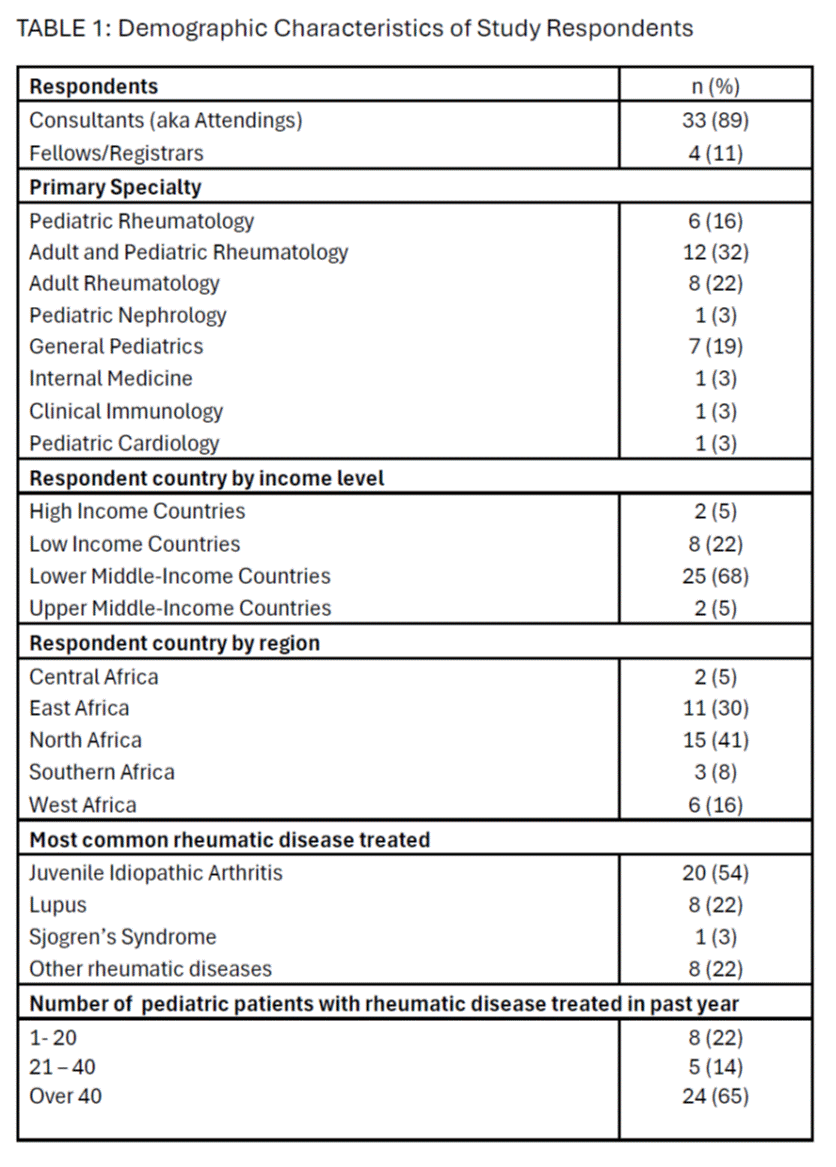
Results: Response rate was 74% (38 of 50). Most respondents were attendings (89%), from Lower Middle-Income Countries (LMIC; 68%), and from North Africa (41%) (Table 1). All physicians had experience in treating PRDs, including 30% of respondents who lacked formal training in rheumatology. ANA testing was needed > 10 times a month (46%). The cost of ANA testing was high at $20 – 60/test (60%). Over 80% of respondents had little to no difficulty collecting and centrifuging venous blood for ANA testing. However, only 31% found it easy to ship samples to a lab within 1 – 2 days of sample collection. There was difficulty in processing ANA testing by ELISA which was worse in the Southern (100%), and West regions (83%) (p=0.03). Over 70% of respondents had difficulty receiving results of ANA testing quickly enough to support patient care which was worse with lower income countries and LMIC compared to others (p = 0.04); and in West and Central/East regions compared to others (p = 0.03). Even with local laboratories available, ANA testing was not readily available in public hospitals and so samples were often sent overseas.
Conclusion: Our results provide highly desired quantitative evidence of the urgent need to improve access to affordable, and robust ANA testing in LRCs in Africa. Specifically, our results highlight critical barriers to ANA testing that will inform successful scaling of testing methods and future research and intervention efforts that would improve early diagnosis of rheumatic conditions in children, adolescents and young adults.Disclosures: Supported by the Cincinnati Children’s Hospital Medical Center Faculty International Research Excellence (FIRE) Award.
To cite this abstract in AMA style:
Ogbu E, Migowa A, Sahay R, Sharma D, Vega-Fernandez P, Henrickson M, Omalla E, Faleye A, Hamdi W, Brunner H. Overcoming Barriers to ANA Testing in Pediatric Populations in Africa [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/overcoming-barriers-to-ana-testing-in-pediatric-populations-in-africa/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/overcoming-barriers-to-ana-testing-in-pediatric-populations-in-africa/