Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Data on Immune Checkpoint inhibitor (ICI) use in patients with previously diagnosed inflammatory muscle disease (IMD) is limited as these patients were excluded from clinical trials. There is a concern that use of ICIs in patients with pre-existing IMD may have flares of their disease, increased morbidity, and/or higher mortality with ICI treatment. We identified all patients diagnosed with IMD prior to ICI infusion in the Veterans Health Administration (VHA) and analyzed these patients’ demographics, diagnoses, laboratory findings, clinical course, and mortality in comparison to other Veterans treated with ICIs.
Methods: This analysis used data from the VHA Corporate Data Warehouse (CDW) for demographics, ICD codes, and pharmacy information, VA cancer registry for cancer diagnosis, and all-cause mortality from the Death Ascertainment File (DAF). Veterans who received one or more ICI infusions between 6/6/2011 and 2/14/2023 were evaluated as potential IMD cases if they had two or more myositis ICD-9 or ICD-10 codes associated with a rheumatology or neurology clinic visit at least 30 days apart and both visits occurred prior to the ICI treatment. Cases meeting these criteria underwent electronic medical record review using the Compensation and Pension Record Interchange (CAPRI) to determine if the case met American College of Rheumatology (ACR) criteria for IMD. Demographic features and mortality of confirmed cases were compared to patients receiving ICI without pre-existing IMD. IMD cases were also reviewed to determine if patients developed a myositis flare.
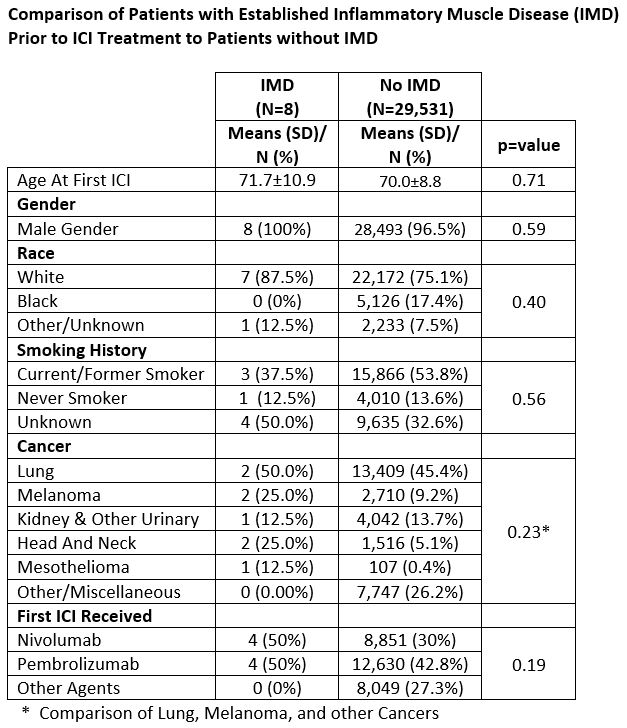
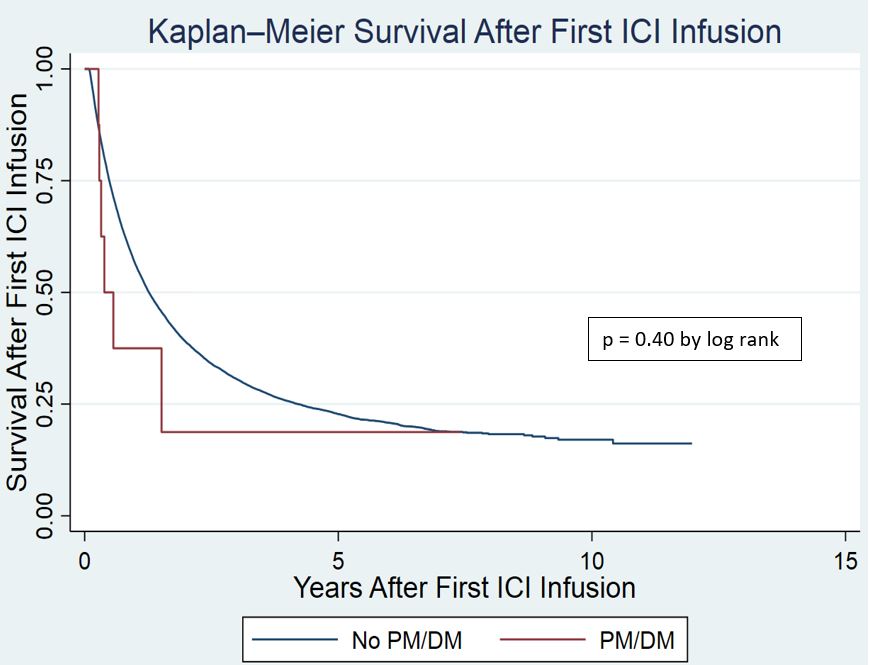
Results: We identified 29,539 patients who received at least one ICI infusion in the VHA. Of these patients, 13 met IMD screening criteria with 8 patients having confirmed IMD by ACR criteria (dermatomyositis 3, polymyositis 2, anti-synthetase syndrome 2, and RA/myositis overlap 1). All patients were white males with an average age of 71.7 years at the time of first ICI infusion. Primary cancer diagnoses were lung – 2, melanoma – 2, head and neck – 2 kidney/other urinary – 1, and mesothelioma – 1. Four patients were treated with nivolumab and four patients with pembrolizumab (Table 1). Diagnosis of IMD was made on average 3.1 years prior to treatment with ICI. One patient with dermatomyositis had an IMD flared after two doses of ICI and was treated successfully with steroids and IVIG. No IMD flares were identified in the other seven patients. There was no difference in Kaplan Meier Survival Analysis for IMD patients in comparison to patients treated with ICI without IMD (p=0.40 by log rank test) (Figure 1).
Conclusion: Overall mortality in these 8 patients with IMD treated with an ICI was similar to other ICI treated Veterans. Among the 8 IMD patients, only one developed an IMD flare after ICI treatment that responded to glucocorticoids and IVIG therapy. These findings suggest that ICI therapy can be considered in IMD patients with close monitoring. Future research is needed to better understand the risk of IMD flare and outcomes in patients with established IMD treated with ICIs.
To cite this abstract in AMA style:
Krutko D, Rubino S, Sauer B, Rojas Jr J, Kunkel G, Walsh J, Patel S, Cannon G, Braaten T. Outcomes of Immune Check Point Inhibitor Use in US Veterans with Pre-Existing Inflammatory Muscle Disease [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/outcomes-of-immune-check-point-inhibitor-use-in-us-veterans-with-pre-existing-inflammatory-muscle-disease/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-of-immune-check-point-inhibitor-use-in-us-veterans-with-pre-existing-inflammatory-muscle-disease/