Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The window of opportunity to address early rheumatoid arthritis (RA) in patients (pts) is well documented. However early aggressive (agr) RA needs further study to identify optimal treatment patterns. This study compares outcomes of early agr RA to early non-agr patients on their first DMARD.
Methods: Pts>18 years and clinically diagnosed with RA in the JointMan database between 1 Jan 2009 and 31 Mar 2017 were included. Test pts had erosions on imaging and were positive for either anti-cyclic citrullinated peptide (ACPA) or rheumatoid factor (RF) within 6 months of their diagnosis. Controls had non-agr early RA with no erosions on imaging and were RF- and ACPA-.
Outcomes using composite disease activity metrics (DAM – DAS28, DAS28-CRP, SDAI, CDAI, and Rapid3) and 8 clinical metrics (tender & swollen joint counts, patient global, pain, physician global physician disease, ESR, and CRP) were tracked at baseline (first DMARD initiation), 6, 12, and 18 months follow up. Index date was the date of the first DMARD. Baseline was defined as the encounter immediately prior to the index date, but no greater than 12 months. Percentage change in each metric from baseline was calculated and differences between test and control (normally distributed) were compared with Welch’s T-Test (significance level of 0.05).
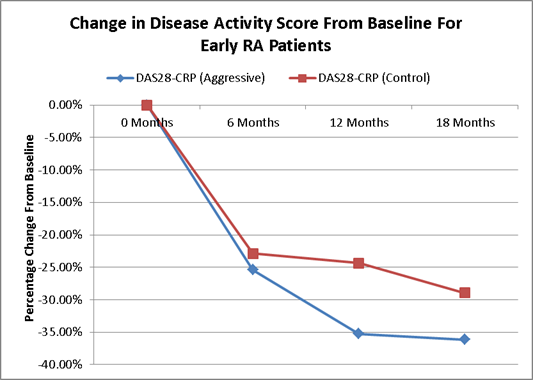
Results: 618 patients were included in the analysis, 177 (28.6%) were test. Compared to controls, test pts were older (60 vs 53 years), but less likely female (71.8 vs 77.8%). There was significant difference between test and controls at baseline for ESR, CRP and all DAMs (p=0.034, 0.013, and <0.022 respectively) except Rapid3. The percentage change was significant between cohorts at 18 months for all DAMs (p<0.0001); DAS28-CRP had the greatest difference in percentage change (test -36.14 vs -28.94%), while the average swollen joint count was significantly different at all time points.
Conclusion: Early agr RA is distinct both at baseline and in its progression to that of non-agr RA. Close clinical monitoring may be necessary to ensure agr pts respond to therapy.
|
Disease Activity Metric
|
Baseline
|
6 Months
|
12 Months
|
18 Months
|
|
DAS28-CRP |
0.002
|
0.027
|
0.525 |
0.0001
|
|
DAS28 |
0.002
|
0.168 |
0.415 |
0.0001
|
|
CDAI |
0.022
|
0.066
|
0.979 |
0.0001
|
|
SDAI |
0.003
|
0.224 |
0.902 |
0.0001
|
|
Rapid3 |
0.774 |
0.734 |
0.551 |
0.0001
|
|
Tender Joint Count |
0.280 |
0.639 |
0.552 |
0.0001
|
|
Swollen Joint Count |
0.000
|
0.009
|
0.029
|
0.0001
|
|
Patient Global |
0.963 |
0.823 |
0.602 |
0.0001
|
|
MD Global |
0.152 |
0.710 |
0.675 |
0.0001
|
|
MD Disease |
0.129 |
0.724 |
0.832 |
0.0001
|
|
Patient Pain |
0.524 |
0.707 |
0.490 |
0.0001
|
|
ESR |
0.034
|
0.226 |
0.248 |
0.0001
|
|
CRP |
0.013
|
0.222 |
0.039 |
0.0001
|
|
The p-values from Welch’s T-Test comparing early aggressive vs early non-aggressive RA. |
||||
To cite this abstract in AMA style:
Knapp K, Mueller E, Craig G. Outcomes in Real-World Patients with Early Aggressive Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/outcomes-in-real-world-patients-with-early-aggressive-rheumatoid-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-in-real-world-patients-with-early-aggressive-rheumatoid-arthritis/