Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Ongoing debate exists regarding the optimal sequence of tumor necrosis factor inhibitors and Janus kinase inhibitors (JAKis) in patients with rheumatoid arthritis (RA) as first-line biologic or targeted synthetic disease-modifying antirheumatic drug (b/tsDMARD) therapy following conventional therapies.The aim of this study was to describe baseline characteristics, effectiveness, persistency, and treatment patterns among first-line b/tsDMARD-naive initiators of etanercept (ETN), adalimumab (ADA), or JAKis (tofacitinib, baricitinib, and upadacitinib).
Methods: Data on patients who initiated b/tsDMARD from 11/2012 to 6/2021 were obtained from the CorEvitas RA Registry, a prospective, multicenter, observational, disease-based registry. Patients ≥18 years with rheumatologist-diagnosed RA and 6- and/or 12-months’ (M) follow-up were included. We report descriptive statistics at baseline, persistency on therapy, escalation/de-escalation of therapy, details on patterns of drug switching, and effectiveness outcomes using regression models adjusted for baseline covariates (demographic/socioeconomic/lifestyle characteristics, comorbidities, medication history, disease activity, and patient-reported outcomes). Outcomes were evaluated at 6M and 12M follow-up.
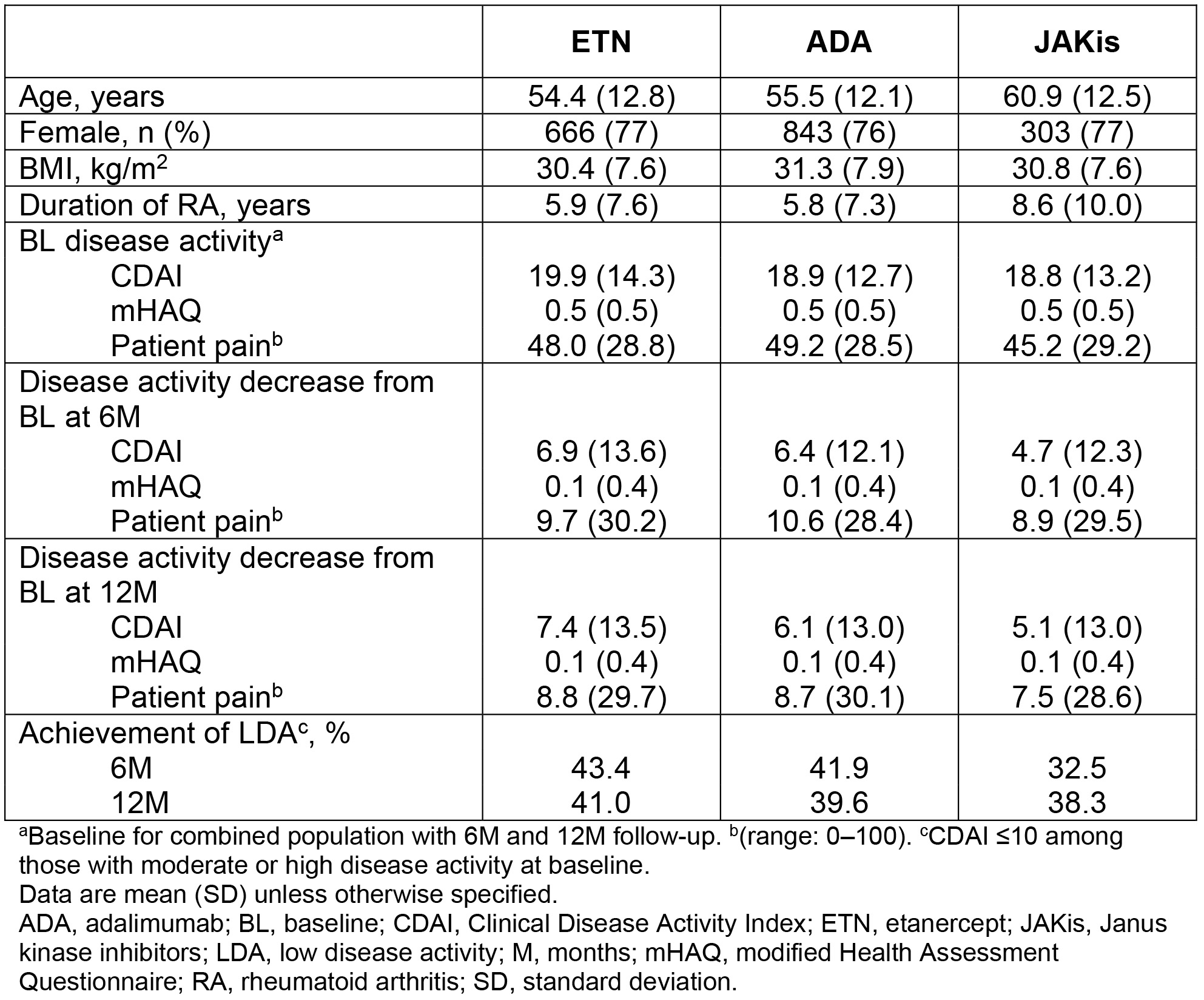
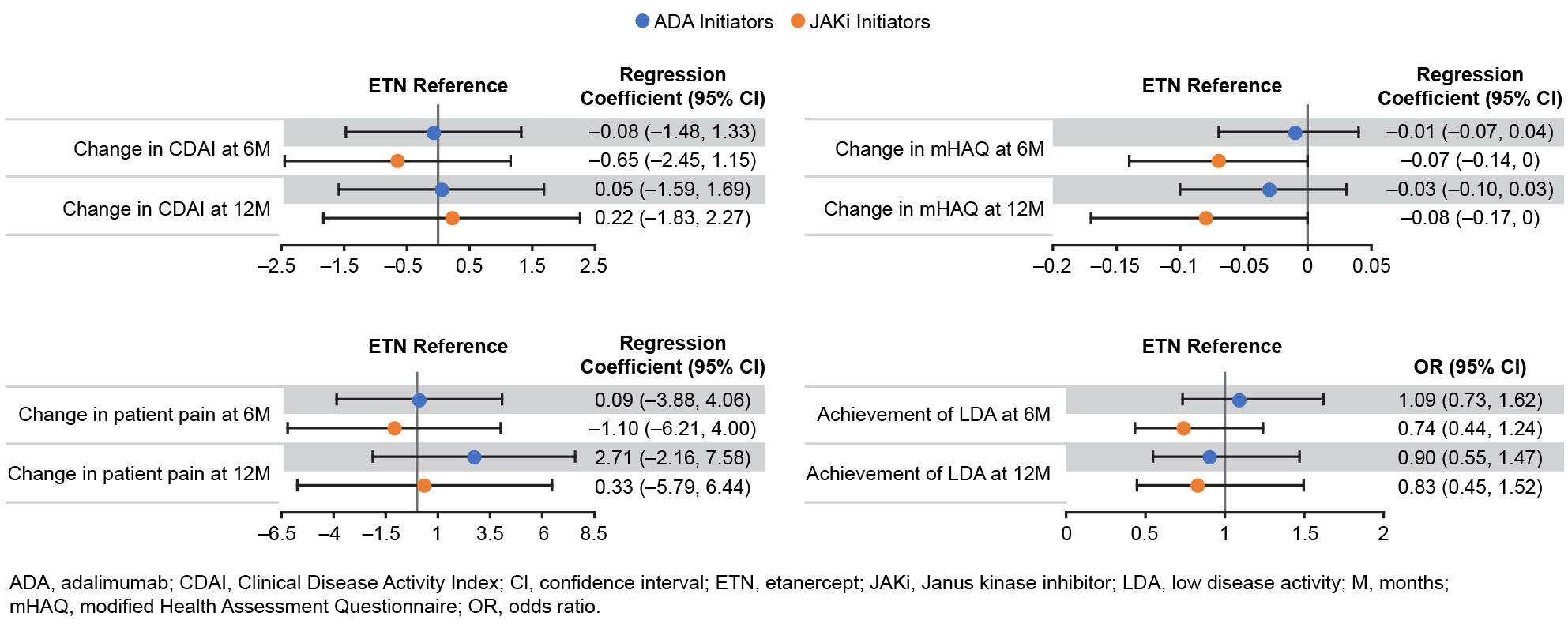
Results: First-line initiators of ETN, ADA, and JAKis with baseline and follow-up visits were identified: 803, 984, and 361 patients at 6M, respectively; 589, 749, and 264 patients at 12M, respectively. Baseline characteristics were similar among ETN, ADA, and JAKi initiators with the exception of disease duration, which was longer among first-line JAKi initiators (mean, 8.6 y) versus ETN (5.9 y) and ADA (5.8 y) initiators. Unadjusted mean improvement in Clinical Disease Activity Index (CDAI) was generally similar between groups at 6M and 12M (Table). Adjusted effectiveness results were similar at 6M and 12M (Figure). At 6M, 68% of ETN, 69% of ADA, and 67% of JAKi initiators remained on the same therapy; at 12M, 53% of ETN, 57% of ADA, and 57% of JAKi initiators remained on the same therapy. The frequency of switching to another b/tsDMARD was similar across initiators.
Conclusion: In this real-world study in patients initiating first-line b/tsDMARD therapy with ETN, ADA, or JAKis, we did not observe differences in clinical effectiveness/patient-reported outcomes and treatment persistency at 6M and 12M after treatment initiation.
To cite this abstract in AMA style:
Pappas D, O’Brien J, Guo L, Shan Y, Baker J, Kricorian G, Stryker S, Collier D. Outcomes in Patients with Rheumatoid Arthritis Initiating Therapy with Etanercept, Adalimumab, or Janus Kinase Inhibitors [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/outcomes-in-patients-with-rheumatoid-arthritis-initiating-therapy-with-etanercept-adalimumab-or-janus-kinase-inhibitors/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-in-patients-with-rheumatoid-arthritis-initiating-therapy-with-etanercept-adalimumab-or-janus-kinase-inhibitors/