Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Multisystem inflammatory syndrome in children (MIS-C) is a clinical entity distinct from primary COVID-19 infection that resembles Kawasaki disease (KD) and toxic shock syndrome. Consensus treatment guidelines for MIS-C have been established based on expert review. Anakinra has been used in refractory cases of MIS-C, but has not been studied in-depth.
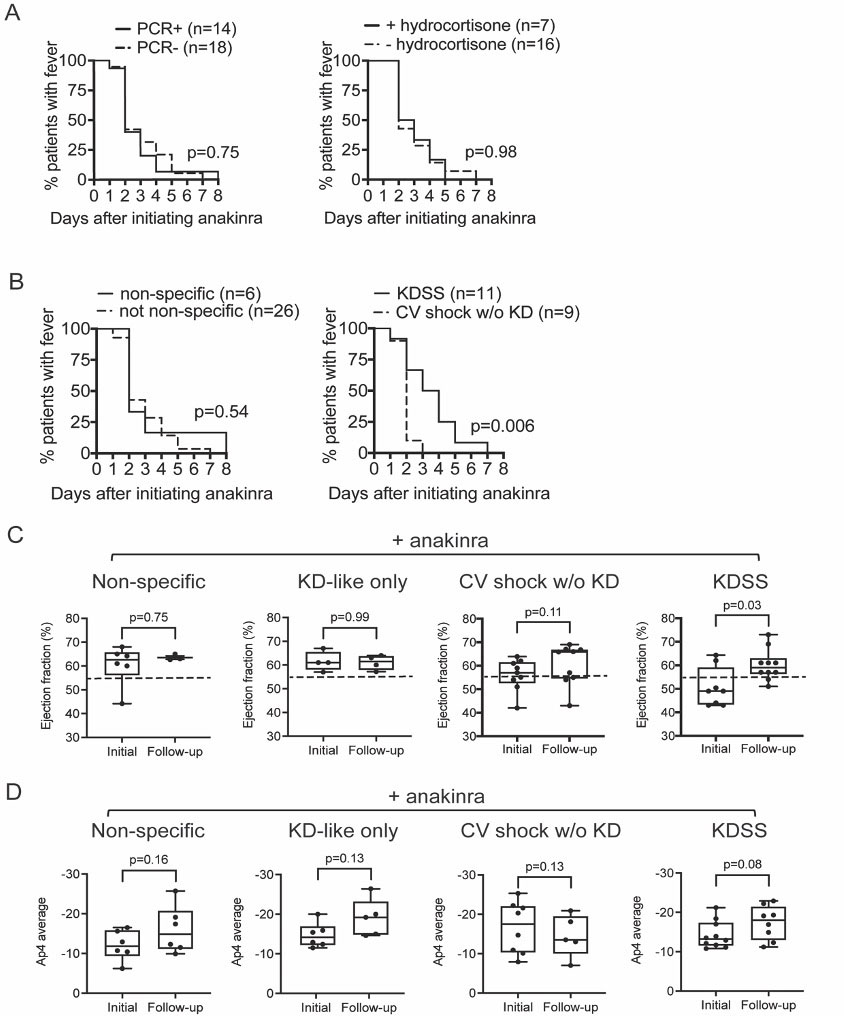
Methods: A single center retrospective cohort study was conducted of all MIS-C patients hospitalized at CNH from May 15 to November 15, 2020. Diagnostic and therapeutic data were extracted from the electronic health record. Forty-six patients met the CDC case definition for MIS-C and were further stratified by the presence/absence of KD-like features and CV shock. MIS-C patients with neither KD-like features nor CV shock were termed non-specific, while those with both were termed Kawasaki disease shock syndrome (KDSS).
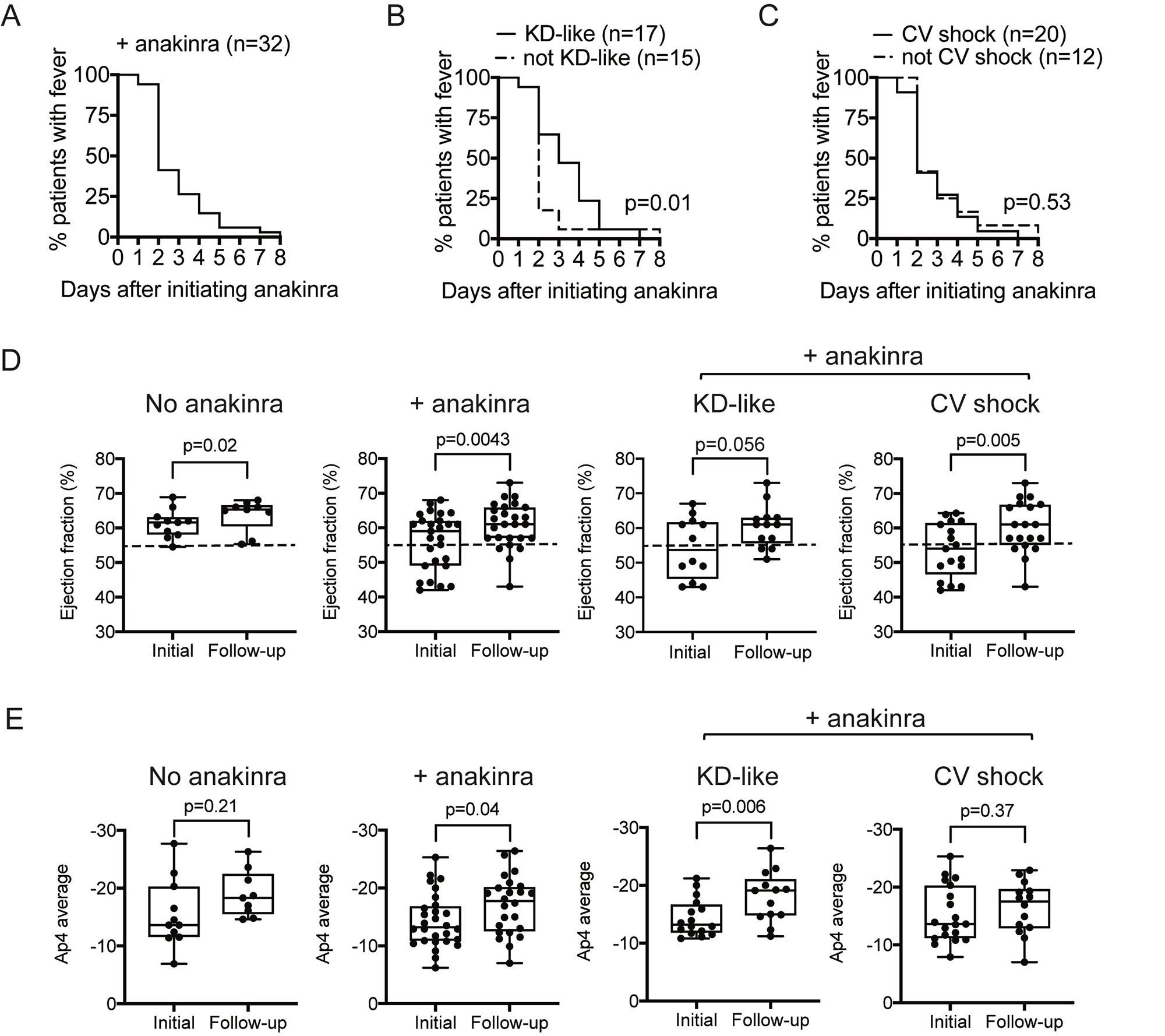
Results: Of the 46 MIS-C patients included in the study, all received IVIG, 14 (30%) received IVIG monotherapy and 32 (70%) received IVIG+anakinra. No mortality was observed, and no statistically significant differences in adverse reactions were found. The median initial dose of anakinra was 7.9 mg/kg/day to a maximum dose of 9.5 mg/kg/day, with a duration of 9.8 days. Most CV shock patients received anakinra (+anakinra 63% versus -anakinra 21%, p=0.02), which were prescribed at higher doses (+CV shock 9.8 mg/kg/day versus -CV shock 8.0 mg/kg/day, p=0.003) and for a longer duration (+CV shock 10.6 days versus -CV shock 7.1 days, p=0.002). In patients who received IVIG+anakinra, >50% defervesced by day 2 of treatment (figure 1A-C). Among patients receiving IVIG+anakinra, KD-like symptoms was associated with a prolonged time to defervescence (figure 1B). MIS-C patients treated with IVIG+anakinra showed improvement in LVEF (59.0% versus follow-up 62.5%, p=0.004) (figure 1D) and Ap4 cardiac strain (median initial -13.2 versus follow-up -17.8, p=0.04) (figure 1E). While improvements in LVEF with IVIG+anakinra remained significant in CV shock and KDSS subgroup analysis (figure 1D and figure 2C), improvement in cardiac strain was significant in those with KD-like features (median initial -3.2 versus follow-up -19.1, p=0.006) (figure 1D), but not CV shock (p=0.37) (figure 1D and 2D).
Conclusion: Treatment of MIS-C with anakinra was associated with resolution of symptoms, including fever and cardiovascular dysfunction, with no significant adverse events. Our findings suggest an alternative strategy associated with resolution of MIS-C-associated hyperinflammation, and may be useful in MIS-C patients for whom steroids may be unfavorable or contraindicated.
To cite this abstract in AMA style:
Brian D, Redmond C, Gotschlich E, Sule S, Ronis T, Vazzana K, Sherman M, Connor R, Bosk A, Dham N, Harahsheh A, Wells E, DeBiasi R, Srinivasalu H. Outcomes and Safety of Anakinra for the Treatment of Multisystem Inflammatory Syndrome in Children [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/outcomes-and-safety-of-anakinra-for-the-treatment-of-multisystem-inflammatory-syndrome-in-children/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-and-safety-of-anakinra-for-the-treatment-of-multisystem-inflammatory-syndrome-in-children/