Session Information
Date: Monday, November 9, 2020
Title: SLE – Treatment Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Hydroxychloroquine (HCQ) is a cornerstone treatment for several autoimmune diseases including Systemic Lupus Erythematosus (SLE). Recently, concerns arose regarding HCQ shortages for SLE patients, due to its use as a potential COVID-19 treatment. Although some patients may remain well after reducing or stopping therapy, others will have potentially life-threatening complications related to SLE flares. We evaluated if HCQ reduction or discontinuation is associated with increased risk of poor outcomes.
Methods: We analyzed prospective data from the Systemic Lupus International Collaborating Clinics (SLICC) cohort, which includes SLE patients from 33 sites in Europe, Asia, and North America, enrolled within 15 months of diagnosis and followed annually, from 1999 to 2019. In patients receiving HCQ, we identified two sub-cohorts, one who reduced HCQ and one who stopped HCQ. We did not require patients to be in disease remission at the time of HCQ reduction/discontinuation for these analyses. Time zero for these sub-cohorts was the date of the first HCQ reduction/discontinuation. For comparison, we identified a third group of patients remaining on HCQ. A poor outcome was defined as either subsequent need for SLE therapy augmentation (steroids or other immunosuppressives), increase of ≥4 points in the SLE Disease Activity Index-2000 (SLEDAI-2K) or hospitalization for SLE. We estimated adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for poor outcomes among the three HCQ exposure groups. Models were adjusted for demographics and baseline clinical characteristics.
Results: A total of 1460 patients were included (89% female, 52% Caucasian). Patients in the HCQ reduction group contributed 1087 person-years, those discontinuing HCQ contributed 677 person-years, and those maintaining HCQ contributed 1649 person-years. HCQ dose reduction or discontinuation occurred in many patients (40%) with a SLEDAI-2K >4. The crude poor outcome rate was significantly lower in the HCQ maintenance group (31.6 events per 100 person-years, 95% CI 29.0, 34.5) than in the reduction (43.0, 95% CI 39.3, 47.1) and in the discontinuation (43.0, 95% CI 38.3, 48.2) groups. Patients reducing or discontinuing HCQ had higher adjusted HRs for poor outcomes versus those maintaining HCQ (Table 1). Other factors independently associated with poor outcomes included active SLE and use of prednisone or immunosuppressive drugs, all measured at time zero.
Conclusion: Patients reducing or discontinuing HCQ are at greater risk of having a poor outcome versus those maintaining the drug. These analyses do not account for reasons HCQ was reduced/discontinued. Regardless, baseline disease activity, prednisone and immunosuppressive drugs were associated with the risk of poor outcomes in SLE patients reducing, discontinuing, or maintain HCQ.
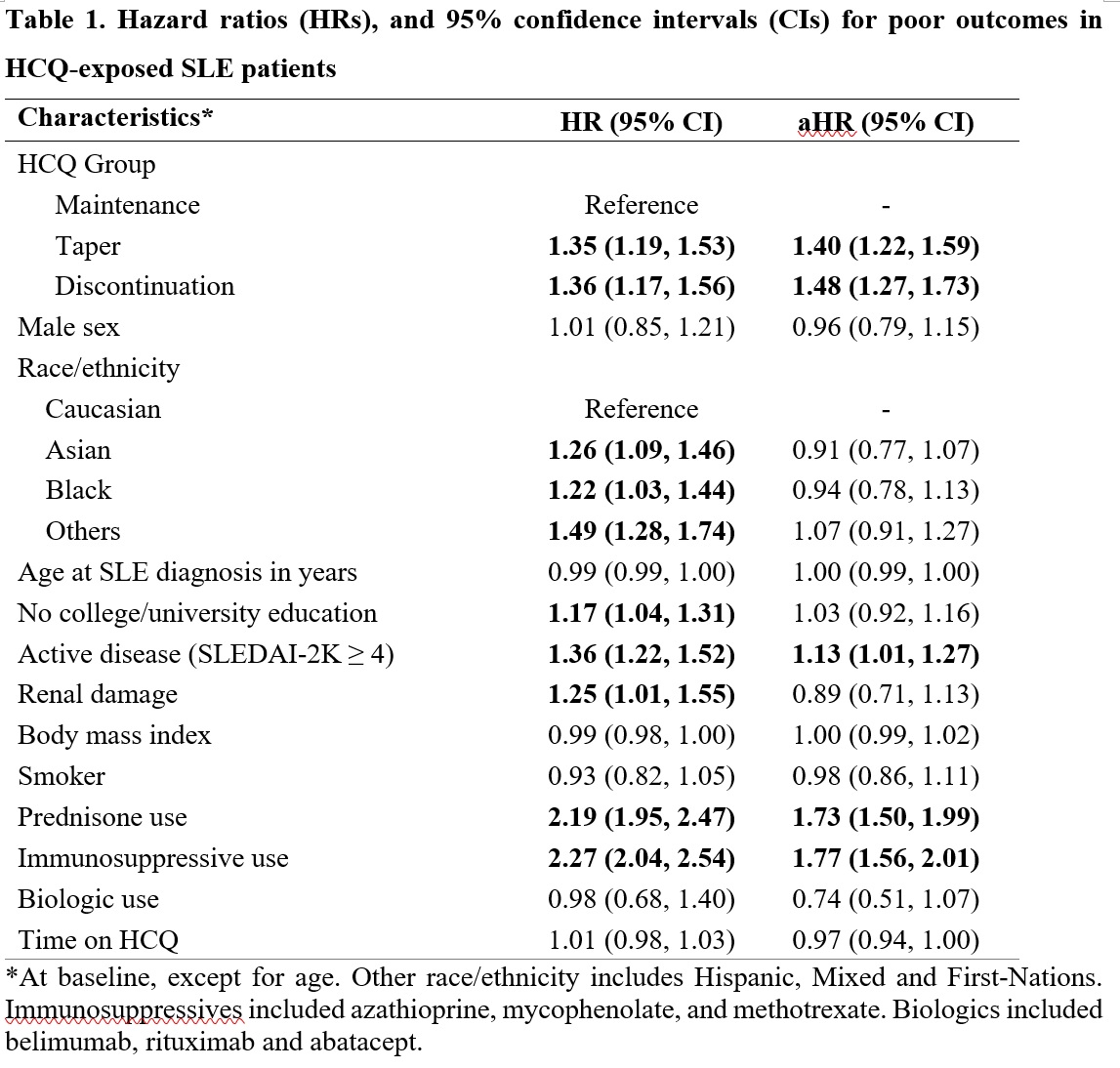 Hazard ratios (HRs), and 95% confidence intervals (CIs) for poor outcomes in HCQ-exposed SLE patients
Hazard ratios (HRs), and 95% confidence intervals (CIs) for poor outcomes in HCQ-exposed SLE patients
To cite this abstract in AMA style:
Almeida-Brasil C, Hanly J, Urowitz M, Clarke A, Ramsey-Goldman R, Gordon C, Petri M, Ginzler E, Wallace D, Bae S, Romero-Diaz J, Dooley M, A. Peschken C, Isenberg D, Rahman A, Manzi S, Jacobsen S, Lim S, Van Vollenhoven R, Nived O, Jönsen A, Kamen D, Aranow C, Ruiz-Irastorza G, Sanchez-Guerrero J, Gladman D, Fortin P, Alarcón G, Merrill J, Kalunian K, Ramos-Casals M, Steinsson K, Zoma A, Askanase A, Khamashta M, Bruce I, Inanc M, Bernatsky S. Outcomes After Hydroxychloroquine Reduction or Discontinuation in a Multinational Inception Cohort of Systemic Lupus [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/outcomes-after-hydroxychloroquine-reduction-or-discontinuation-in-a-multinational-inception-cohort-of-systemic-lupus/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcomes-after-hydroxychloroquine-reduction-or-discontinuation-in-a-multinational-inception-cohort-of-systemic-lupus/
