Session Information
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Environmental and occupational exposures have been epidemiologically linked to the development of rheumatoid arthritis-associated interstitial lung disease (RA-ILD). Previous investigation and modeling have also suggested that airborne inflammatory biohazards can trigger post-translational modifications (PTMs) capable of stimulating immune response against neo-epitopes. For PTMs such as citrullination, this immune response is strongly associated with HLA-DRB1*0401 and other shared epitope alleles. To further elucidate mechanisms linking environmental insults, immunogenetic background, and PTM-targeted immune responses to the development of RA-ILD, we utilized the established organic dust extract (ODE) airway inflammatory exposure model in HLA-DR4 transgenic (DR4 TG) versus C57BL/6 wild type (WT) mice.
Methods: WT and DR4 TG mice were exposed to intranasal inhalation of ODE (12.5%) versus sterile saline (n=5 mice/treatment group/strain) on a daily basis for 4 weeks. Following this 4 week treatment period, bronchoalveolar lavage fluid (BALF) was collected for cellular and cytokine analysis. Serum was also collected for ELISA-based assessment of autoantibodies targeting citrullinated and MAA (malondialdehyde-acetaldehyde)-modified substrate antigens. Lung tissue was subjected to immunofluorescence (IF) staining using antibodies recognizing peptidyl-citrulline (clone F95, EMDMillipore), MAA-modified proteins (rabbit polyclonal), and vimentin (Bioss). Quantitative scoring of fluorescence in combined regions of interest was compared using one-way ANOVA and Student’s t-tests.
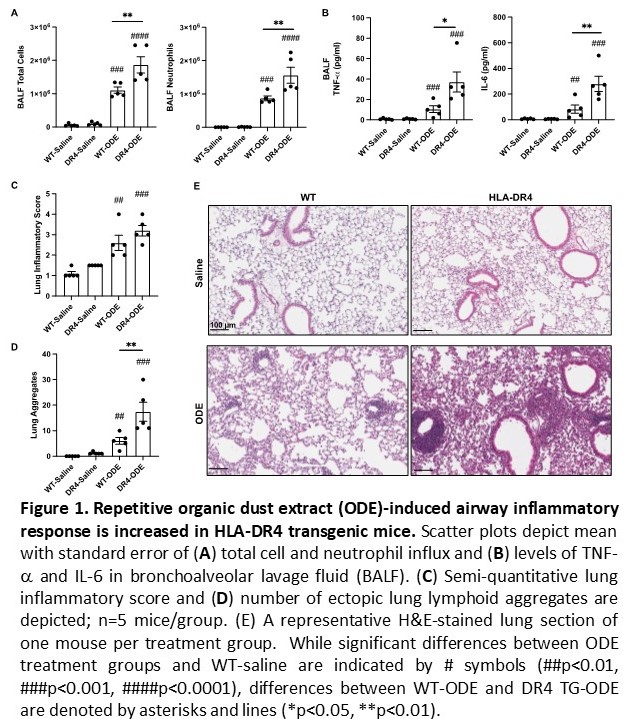
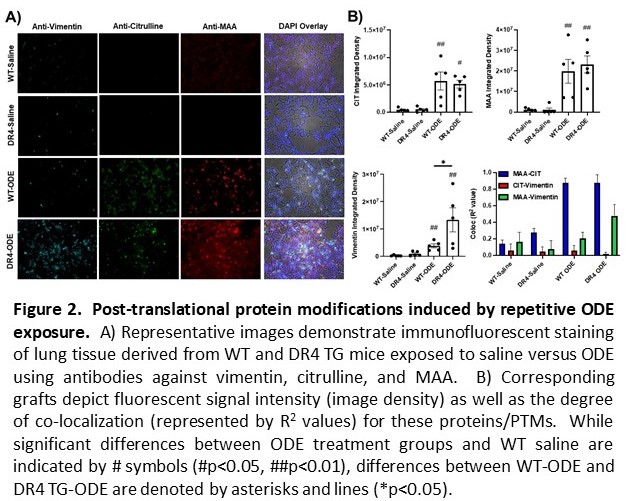
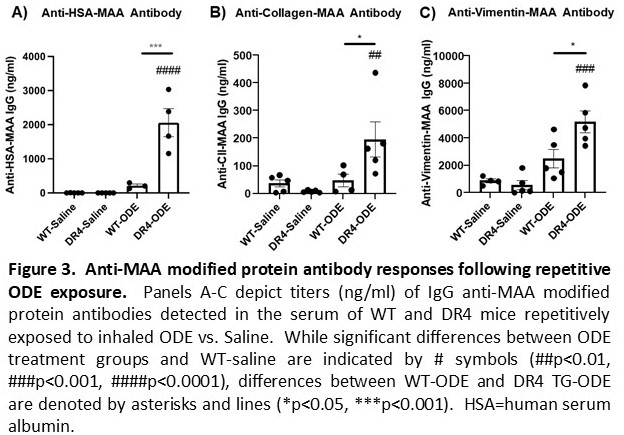
Results: ODE-induced airway cellular influx driven by neutrophils was significantly increased in DR4 TG versus WT mice, with corresponding increases in BALF levels of TNF-a and IL-6 (p< 0.01 for both cytokines, DR4 TG-ODE vs. WT-ODE) (Figure 1A-B). Lung histopathology demonstrated an increased number of ectopic lymphoid aggregates as well as overall lung inflammation in DR4 TG relative to WT mice after repetitive ODE exposure (Figure 1C-E). Corresponding IF staining of lung tissue showed that PTMs such as citrullination and MAA modification were strikingly enhanced in both DR4 TG-ODE and WT-ODE mice (Figure 2). Additional analysis revealed increased co-localization of vimentin and MAA staining that was most pronounced in DR4 TG mice (Figure 2A-B). Custom ELISAs demonstrated an increase in anti-MAA modified protein antibodies (including anti-MAA-vimentin antibodies) following ODE treatment that was amplified in DR4 TG mice (p< 0.05, Figure 3). Mice demonstrated no evidence of arthritis and had no detectable ACPA.
Conclusion: ODE-induced lung inflammation is more pronounced in DR4 TG relative to WT mice. This disease phenotype is accompanied by enhanced PTM formation and humoral immune responses targeting post-translationally-modified proteins. Overall, these results support a model in which environmental insults trigger PTM formation that, in the selected immunogenetic background of HLA-DR4 TG mice, generates lung-centered, PTM-targeted immune responses and a tissue phenotype sharing immunopathological features with RA-ILD.
To cite this abstract in AMA style:
Poole J, Mikuls T, Nelson A, Gaurav R, Duryee M, Thiele G, England B, Ascherman D. Organic Dust Exposure Induces Post-translational Protein Modifications and a HLA-DR4-dependent Pro-inflammatory Lung Phenotype in a Murine Model of Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/organic-dust-exposure-induces-post-translational-protein-modifications-and-a-hla-dr4-dependent-pro-inflammatory-lung-phenotype-in-a-murine-model-of-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/organic-dust-exposure-induces-post-translational-protein-modifications-and-a-hla-dr4-dependent-pro-inflammatory-lung-phenotype-in-a-murine-model-of-interstitial-lung-disease/