Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Olokizumab (OKZ) is a direct interleukin-6 inhibitor for treatment of rheumatoid arthritis and is being investigated in the open-label Phase 2 trial in adolescents with polyarticular-course juvenile idiopathic arthritis (pcJIA). The aim of this study is to assess the pharmacokinetics (PK), effectiveness and safety of OKZ in patients with pcJIA who had inadequate response to methotrexate (MTX).
Methods: Adolescent (12-17 years old) patients with active pcJIA received OKZ in a dose of 64 mg every 4 weeks (q4w) subcutaneously (SC) in open regimen during the main 24-weeks part of the study. The following outcomes were analyzed: JIA American College of Rheumatology 30/50/70% responses (ACR pedi 30/50/70 responses), Juvenile Arthritis Disease Activity Score-71 (JADAS-71), its components, C-reactive protein (CRP), and Childhood Health Assessment Questionnaire (CHAQ), PK parameters. The statistical analysis utilized the method of descriptive statistics. The significance of change from baseline was tested using the Wilcoxon signed-rank test.
Results: Totally 16 patients: 9 [56.3%] girls and 7 [43.8%] boys were enrolled; median (25%; 75%) age at inclusion was 14.0 (13.0; 16.5) years, at pcJIA onset 11.8 (5.4; 13.7) years, and pcJIA duration was 3.4 (0.4; 8.9) years. Concomitant MTX received 12 (75.0%) patients, and 9 (56.3%) patients experienced bDMARDs treatment earlier, of them 8 had ≥2 previous bDMARDS. 43.7% patients were RF-positive and ANA was found in 56.3%.
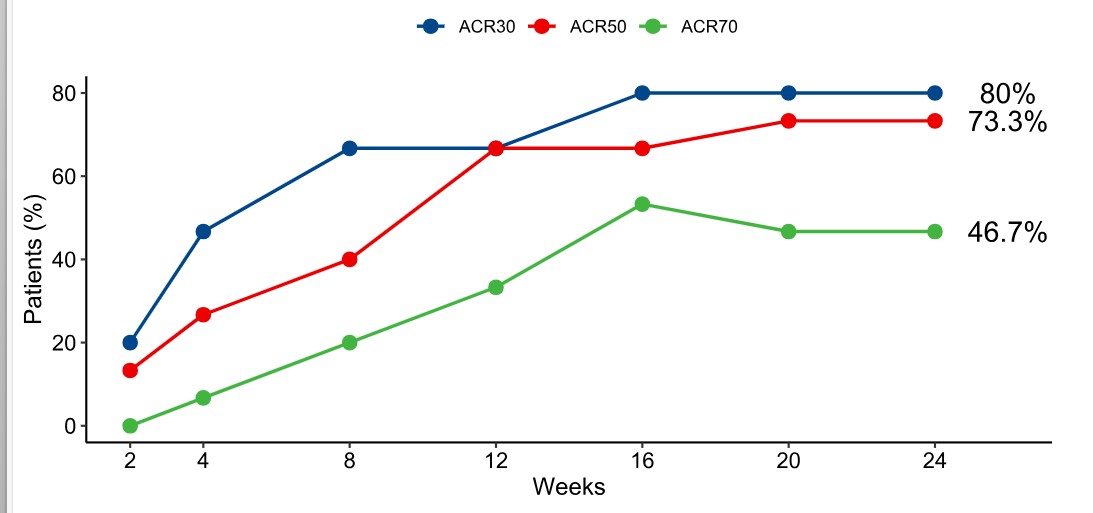
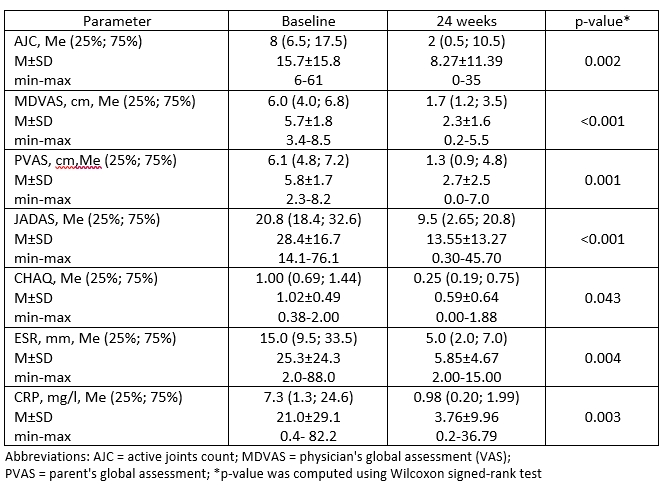
By 24 weeks of treatment, all studied JIA outcomes significantly (p< 0.01) decreased from the baseline: ΔAJC = - 66.7%, ΔJADAS71= - 61.5%, ΔCHAQ= -66.7% (p< 0.05), ΔPVAS= -80.8%, ΔMDVAS= -61.8%, ΔESR= -75.0%, ΔCRP= -81.8% (Table 1). JIA ACR 30/50/70 response was achieved in 80%, 73.3%, 46.7% of patients, respectively (Figure 1). No pcJIA flares were registered. In 5 (33.3%) patients inactive disease status was achieved at week 24. Median Tmax was 168 hours, AUC(0-24) 32309.0 µg*h/ml, Cmax 13.3 µg/ml. Treatment-emergent adverse events (AEs) were reported in 12 (75%) patients. The most frequent AEs were ordinary infections, in 6 (37.5%) patients, all resolved without complications. No Grade 3 or 4 AEs, serious AEs or deaths were reported. There was 1 case of Grade 2 neutropenia, which resolved spontaneously. One patient discontinued treatment at Week 12 due to psoriasis de novo. In one patient local injection reaction (6.2%) was observed.
Conclusion: OKZ in patients with pcJIA was associated with improvement in disease activity and functional health status. Safety was expected for this class of agents, but it seems OKZ has advantages regarding the risk of developing neutropenia.
To cite this abstract in AMA style:
Alexeeva E, Dvoryakovskaya T, Zholobova E, Krekhova E, Raupov R, Bukhanova D, Egorova A, Kuzkina S, Samsonov M, Nikishina I, Kostik M. Olokizumab, a Monoclonal Antibody Against IL-6, in Polyarticular-course Juvenile Idiopathic Arthritis (pcJIA): Results of 24 Weeks of the Phase 2 Open-label Clinical Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/olokizumab-a-monoclonal-antibody-against-il-6-in-polyarticular-course-juvenile-idiopathic-arthritis-pcjia-results-of-24-weeks-of-the-phase-2-open-label-clinical-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/olokizumab-a-monoclonal-antibody-against-il-6-in-polyarticular-course-juvenile-idiopathic-arthritis-pcjia-results-of-24-weeks-of-the-phase-2-open-label-clinical-trial/