Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Prior studies have evaluated the risk factors for difficult-to-treat RA (D2T-RA) but have not applied EULAR’s full criteria for D2T-RA in a longitudinal data set. The objective of our study is to examine the association between multiple demographic, clinical and disease activity factors with the risk of D2T-RA in a cohort of patients with established RA who are being treated with a first line biologic or targeted synthetic disease modifying antirheumatic drug (b/tsDMARD).
Methods: We used data from the longitudinal Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) registry subset to participants treated with their first b/tsDMARD therapy (index date = earliest BRASS visit reporting a first-line b/tsDMARD therapy) and who had at least one follow-up visit. D2T-RA was defined using EULAR 2021 criteria. Using multiple imputation for missing values of potential predictors, we fit Cox proportional hazards (PH) models with stepwise selection based on AIC to identify predictors of D2T-RA at a future study visit. We present hazards ratios (HRs) and 95% confidence intervals for unadjusted and multivariable adjusted models. We checked the PH assumption by evaluating Schoenfeld residuals and log-log plots and tested for multicollinearity. Random Survival Forests models were built to confirm predictors identified in Cox PH models.
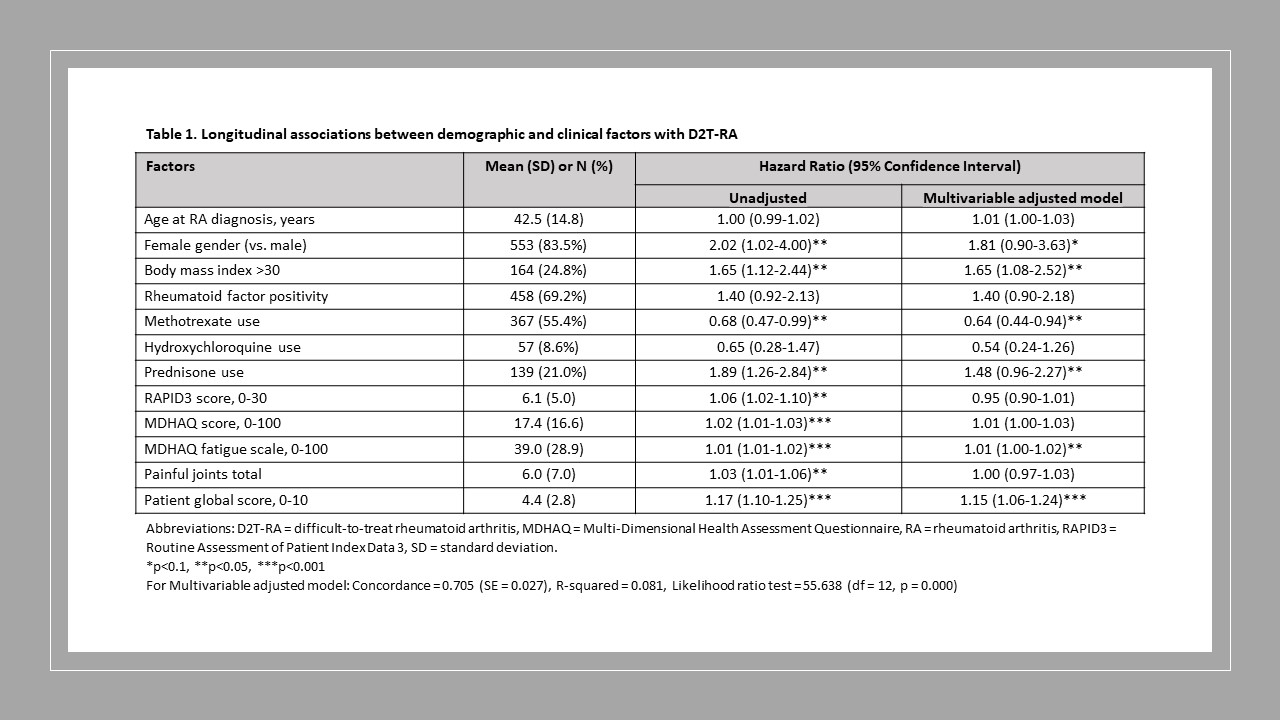
Results: Among 1,581 enrollees in BRASS, we identified 662 (42%) participants currently treated with a first line b/tsDMARD (59% etanercept; 25% adalimumab, 9% infliximab, 7% other). The median (IQR) age was 56 (46-65) years, 84% were female, 91% were non-Hispanic White, and the median (IQR) disease duration was 9 (4-20) years). Over a median follow-up of 60 (IQR 36-120) months, 113 (17%) developed D2T-RA. The final multivariable-adjusted model had a C-index of 0.705. BMI >30 kg/m2 (HR=1.65), concurrent prednisone use (HR=1.48), patient global activity score (HR=1.15 for a one-unit increase), and MDHAQ fatigue scale (HR=1.01 for a five-unit increase) were associated with greater PH of becoming D2T, whereas concurrent methotrexate use was associated with 36% reduced PH of D2T-RA (HR=0.64). Random Survival Forests didn’t improve the performance over Cox PH (C-index=0.636).
Conclusion: In this cohort of 662 participants with established RA being treated with a first-line b/tsDMARD therapy, 17% developed D2T-RA over a median of 60 months. Clinical factors of obesity, prednisone use, and not concurrently taking methotrexate during treatment with a first-line b/tsDMARD were predictive of becoming D2T, along with patient reported outcomes of greater fatigue and disease activity. Future studies should evaluate risk factors for subphenotypes of inflammatory and non-inflammatory D2T-RA.
To cite this abstract in AMA style:
Paudel M, Chitineni S, Li R, Naik C, Shadick N, Weinblatt M, Solomon D. Obesity, Prednisone Use, and Patient-Reported Outcomes Are Predictors of Becoming Difficult-to-Treat in an RA Population Treated with a First-Line Biologic DMARD [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/obesity-prednisone-use-and-patient-reported-outcomes-are-predictors-of-becoming-difficult-to-treat-in-an-ra-population-treated-with-a-first-line-biologic-dmard/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/obesity-prednisone-use-and-patient-reported-outcomes-are-predictors-of-becoming-difficult-to-treat-in-an-ra-population-treated-with-a-first-line-biologic-dmard/