Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
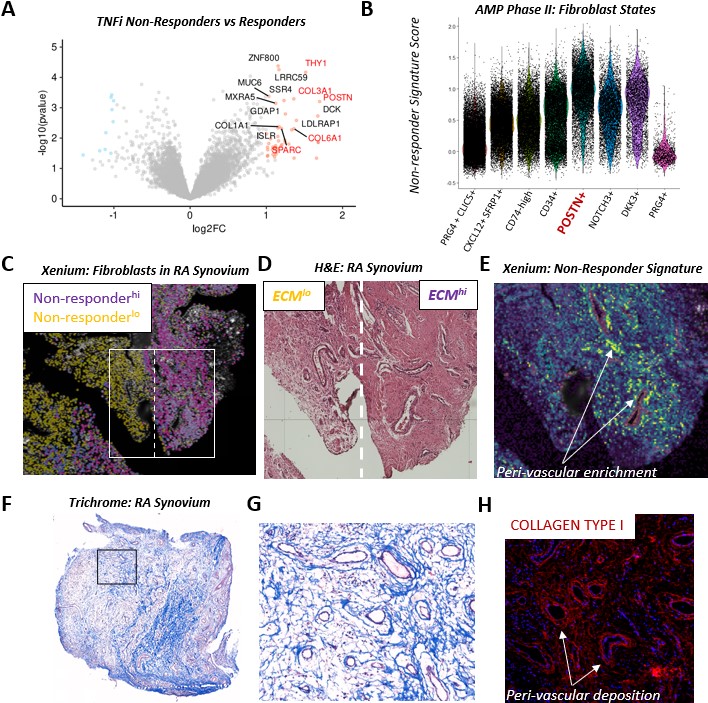
Background/Purpose: In RA, poor treatment response has been linked to a fibroid synovial tissue phenotype, characterized by lack of immune cell infiltration and an expansion of synovial fibroblasts. Recent transcriptomic analysis of synovial tissue from treatment non-responders reveals strong enrichment of a fibrogenic extracellular matrix (ECM) gene signature. Here, we leveraged spatial transcriptomics and in vitro studies to spatially map the treatment non-responder signature in RA synovium and identify key drivers of fibrogenic signaling.
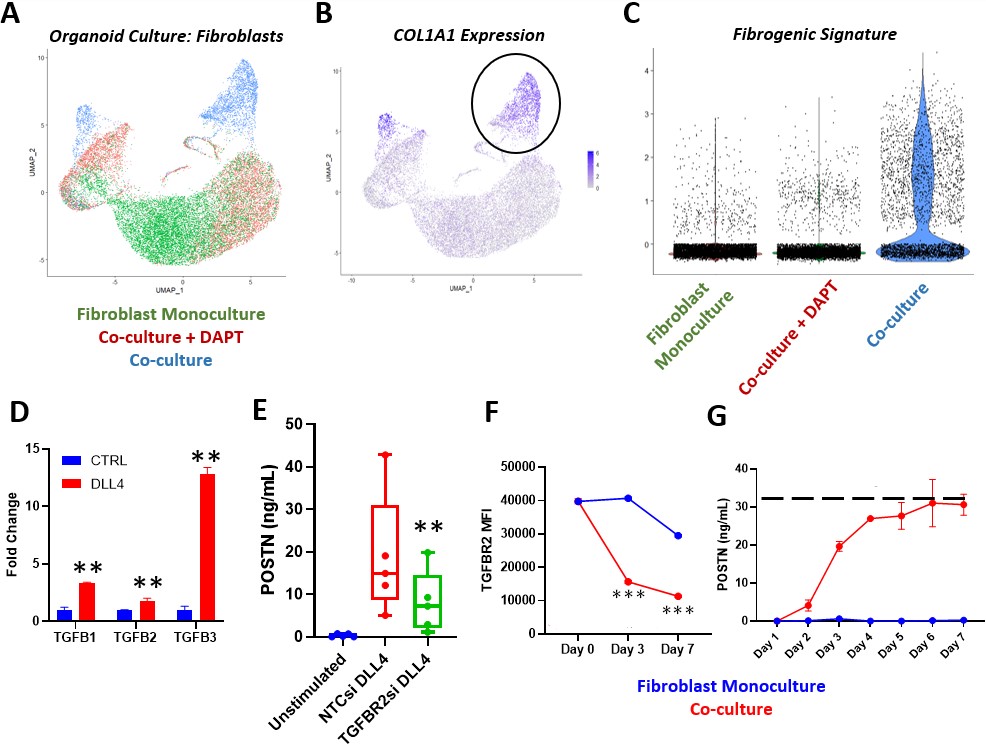
Methods: Synovial FFPE tissue sections (4 RA) were subject to the Xenium in situ workflow. Samples were probed with a custom panel of 50 genes designed to identify the treatment non-responder fibrogenic signature. Trichrome and immunofluorescent staining was performed on additional RA synovial samples. For in vitro experiments, synovial fibroblasts were stimulated with plate-bound Notch ligand DLL4 or co-cultured with endothelial cells (HUVECs). Fibroblasts were subsequently lysed for qPCR or western blot. Media from cultured cells were collected for analysis of ECM production by ELISA.
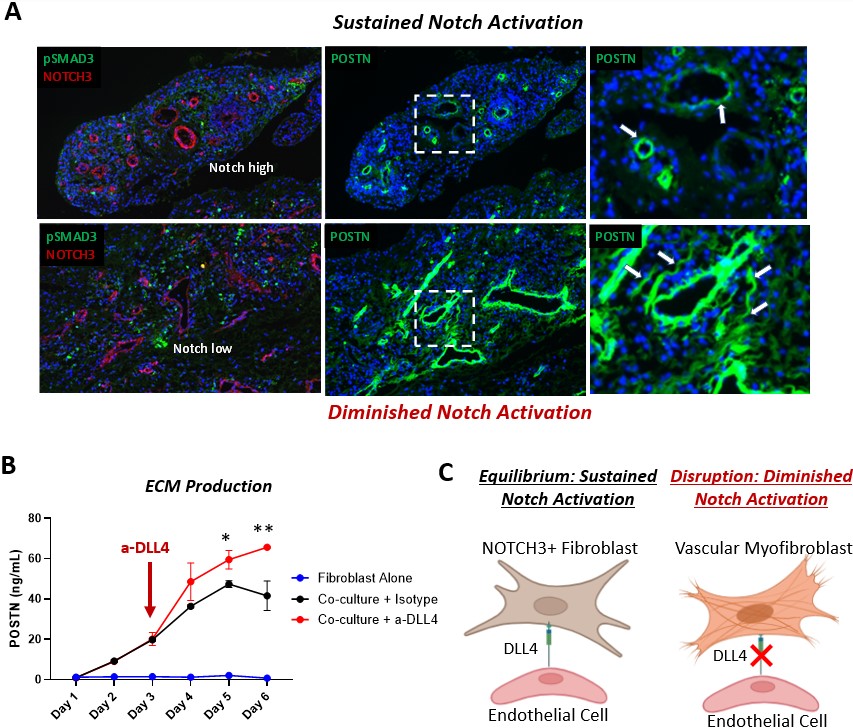
Results: Through transcriptomic analysis of synovial biopsies of treatment naïve RA patients classified by TNFi response status1, we identified a fibrogenic signature that was strongly enriched in TNFi non-responders. Interestingly, the fibrogenic signature enriched in non-responders mapped to a novel POSTN+ sublining fibroblast subset, recently described in the single-cell RA atlas2. The non-responder signatures are found in the perivascular niches in RA, and given the localization of the signature, we hypothesized that Notch signaling was linked to fibrogenic activation. In organoid co-cultures of fibroblasts and HUVECs, we observed an expansion of an ECM expressing fibroblast population that was absent with inhibition of Notch signaling3. DLL4 stimulation of fibroblasts in vitro induced significant TGFβ isoform expression, driving autocrine POSTN production that was diminished by TGFβRII silencing. In co-culture, TGFβRII was downregulated by endothelial-derived Notch in a time-dependent manner, leading to fibroblast tolerization and stabilization of ECM production. Analysis of RA synovium revealed evidence of exuberant fibrogenic activation (pSMAD2/3 and POSTN) in regions with diminishing Notch activation compared to regions with sustained Notch activity. Disruption of endothelial—fibroblast crosstalk with DLL4 blockade in co-cultures led to further induction of fibrogenic ECM production via re-sensitization of fibroblasts to TGFβ.
Conclusion: Here, we defined and spatially mapped a fibrogenic fibroblast cell state associated with treatment resistance. We found that Notch signaling controls fibrogenic activation by modulating both TGFβ isoform and receptor expression. Loss of sustained Notch signaling drives a treatment non-response fibrogenic program. Targeting Notch signaling presents an alternate therapeutic axis for treatment refractory patients.
1. Wang, J. et al. Arthritis Rheumatol. 2022. doi: 10.1002/art.42295
2. Zhang, F et al. Nature. 2023. doi: 10.1038/s41586-023-06708-y
3. Wei, K et al. Nature. 2020. doi: 10.1038/s41586-020-2222-z
To cite this abstract in AMA style:
Bhamidipati K, Gao C, Anufrieva K, Kazerounian S, Madhu R, Tran M, Khedgikar V, Presti S, Jonsson A, Korsunsky i, Wei K. Notch—mediated Fibrogenic Activation Is a Spatial Determinant of RA Treatment Response [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/notch-mediated-fibrogenic-activation-is-a-spatial-determinant-of-ra-treatment-response/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/notch-mediated-fibrogenic-activation-is-a-spatial-determinant-of-ra-treatment-response/