Session Information
Date: Monday, November 18, 2024
Title: Metabolic & Crystal Arthropathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: People with gout need to adhere to medication over time to achieve good outcomes. We assessed self-reported adherence to medication with urate lowering therapy (ULT) 5 years after a treat-to-target intervention and studied how non-adherence was related to baseline demographic and disease variables.
Methods: Patients in the NOR-Gout observational study with a recent gout flare and serum urate >360 µmol/L had attended one year of tight-control visits during escalating urate lowering therapy according to a treat-to-target strategy. Clinical and patient reported data were collected at all time points and a 5-year follow-up including the Medication Adherence Report Scale (MARS-5) questionnaire.
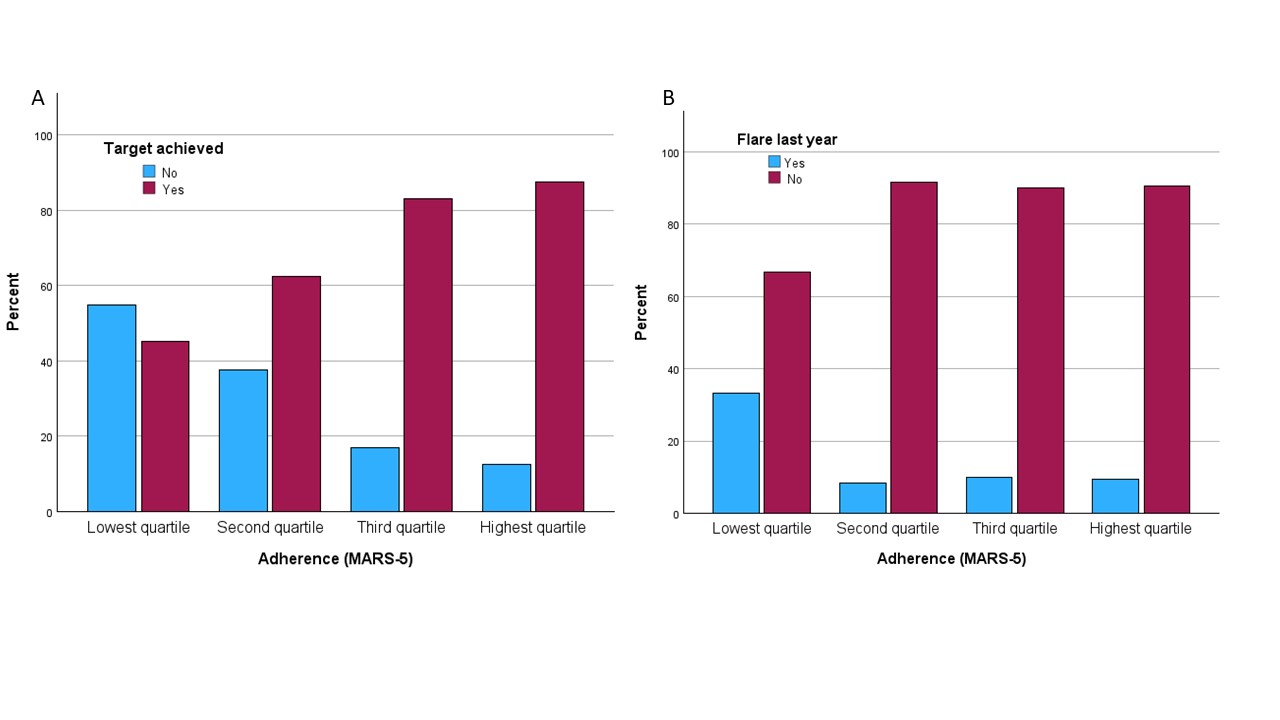
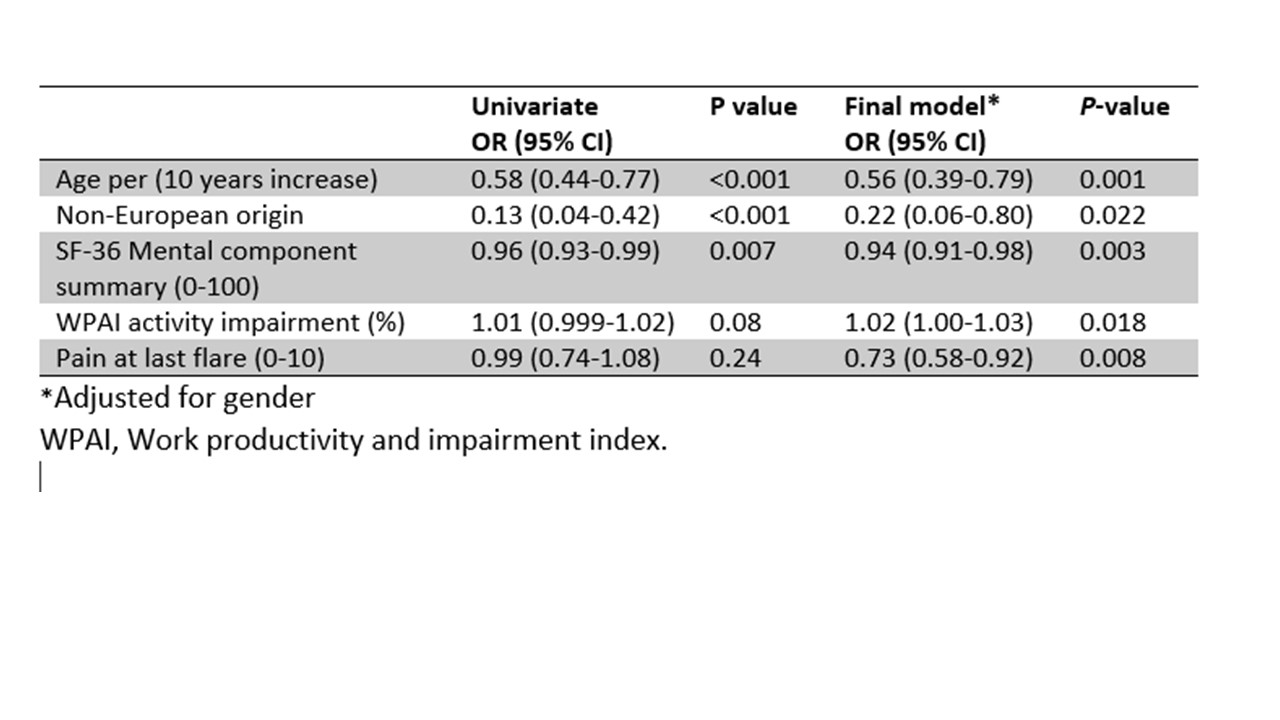
Results: Most of the 163 patients at 5-year follow-up (baseline mean 56.4 years, 95% males) used ULT (95.1%). MARS-5 adherence scores after 5 years were high (median 24, interquartile range 22-25). After 5 years patients in the lowest MARS-5 quartile had, compared to the highest quartile, reached the 5-yr serum urate treatment target less frequently (45.2% vs. 87.5%, P< 0.001) and had more often experienced a flare during the last year of follow-up (33.3% vs. 9.5%, P=0.004)(Figure 1). Baseline lower age, non-European origin, lower mental health scores and less joint pain during the last gout flare were independently associated with non-adherence to medication after 5 years (Table 1).
Conclusion: Patients with gout had, 5 years after a treat-to-target intervention, high self-reported adherence to medication. Non-adherence was related to less frequent serum urate target achievement and more gout flares, and associated with younger age as well as non-European origin. Clinicians should consider these factors to support adherence in gout.
To cite this abstract in AMA style:
Uhlig T, Karoliussen L, Sexton J, Provan S, Haavardsholm E, Dalbeth N, Hammer H. Non-adherence to Urate Lowering Therapy in Gout After 5 Years Is Related to Poor Outcomes – Results from the NOR-Gout Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/non-adherence-to-urate-lowering-therapy-in-gout-after-5-years-is-related-to-poor-outcomes-results-from-the-nor-gout-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/non-adherence-to-urate-lowering-therapy-in-gout-after-5-years-is-related-to-poor-outcomes-results-from-the-nor-gout-study/