Session Information
Date: Sunday, November 12, 2023
Title: (0252–0282) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Japanese patients with connective tissue disease (CTD) are more likely to have interstitial lung disease (CTD-ILD) than those in Western countries and many CTD-ILDs were treated with various immunosuppressive agents (IS). Recently, nintedanib (NTB), a multitargeted tyrosine kinase inhibitor, has been approved for progressive fibrosing ILD (PF-ILD) including CTD-ILD. However, the efficacy of NTB in combination with IS for CTD-associated PF-ILD (CTD-PF-ILD) is unclear. We aimed to clarify the efficacy and safety of NTB in combination with IS for CTD-PF-ILD under real-world clinical settings, as well as biomarkers reflecting therapeutic efficacy.
Methods: CTD-ILD patients who met the criteria for PF-ILD (Flaherty KR, et al. N Engl J Med. 2019) and received NTB at our institution between 2020 and 2022 were included in this retrospective study. Efficacy of NTB treatment was evaluated by changes in forced vital capacity (FVC (%, mL)) at 6–12 months and the monthly change in FVC (ΔFVC (%/M)) before and after NTB administration. As biomarker reflected of ILD activity, the level of serum KL-6 (Krebs von den Lungen-6) were also analyzed. In addition, the effect of concomitant IS on CTD-PF-ILD was evaluated by comparisons of change in FVC and ΔFVC (%/M) between patients who received new IS after the initiation of NTB (Group A) and those who received only NTB (Group B). Safety was analyzed by the occurrence of adverse events (AEs) considered to be associated with NTB within 12 months.
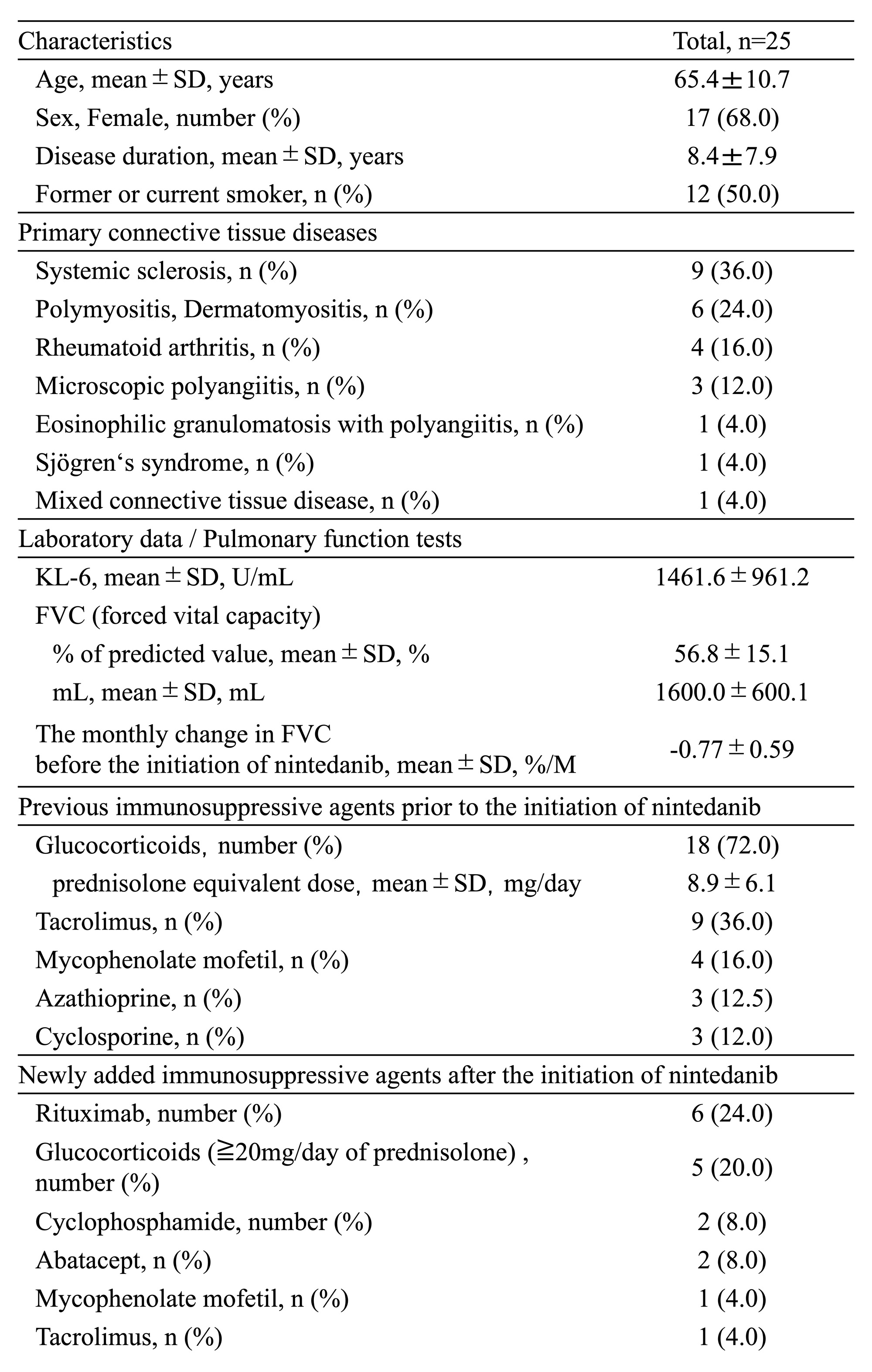
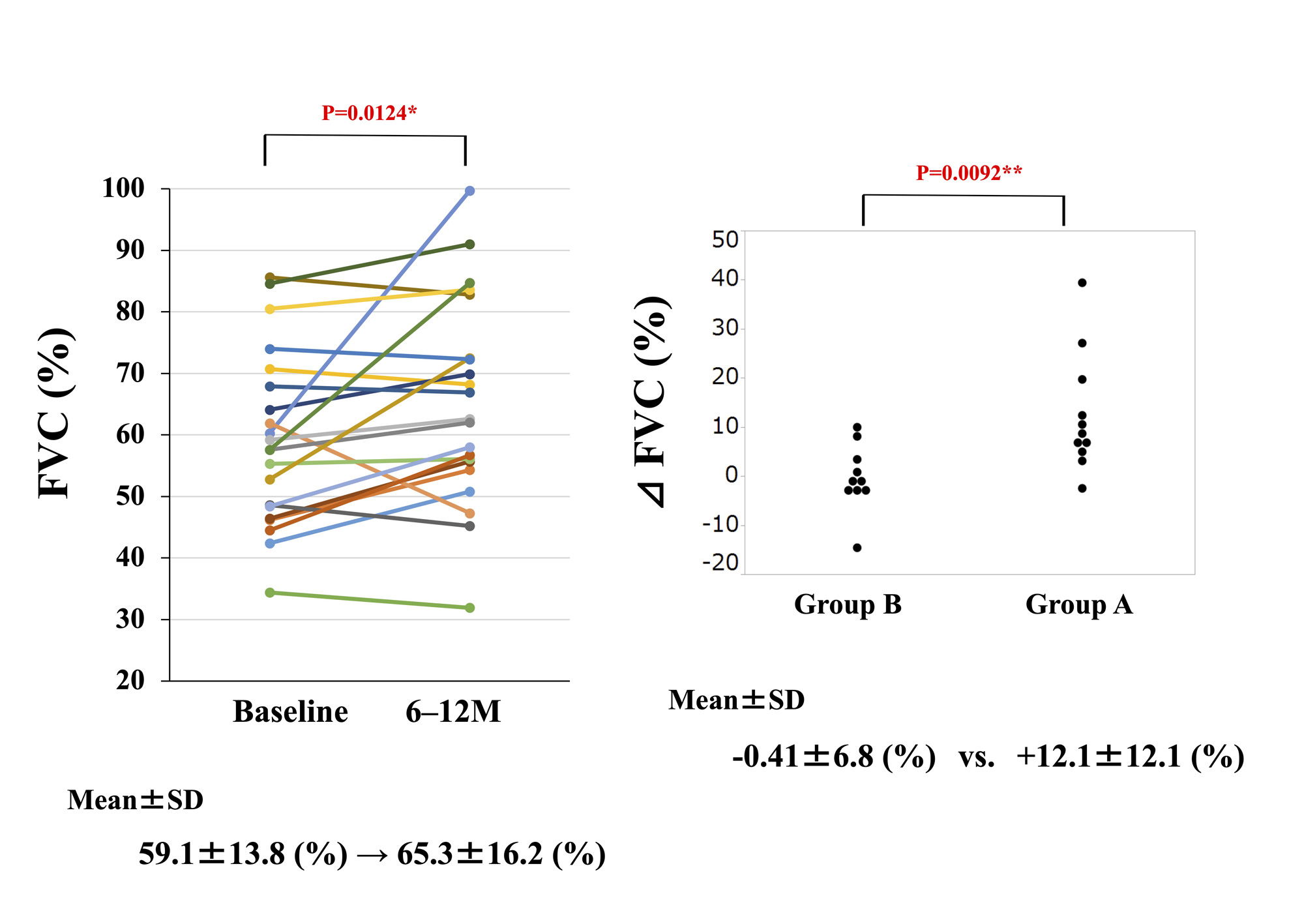
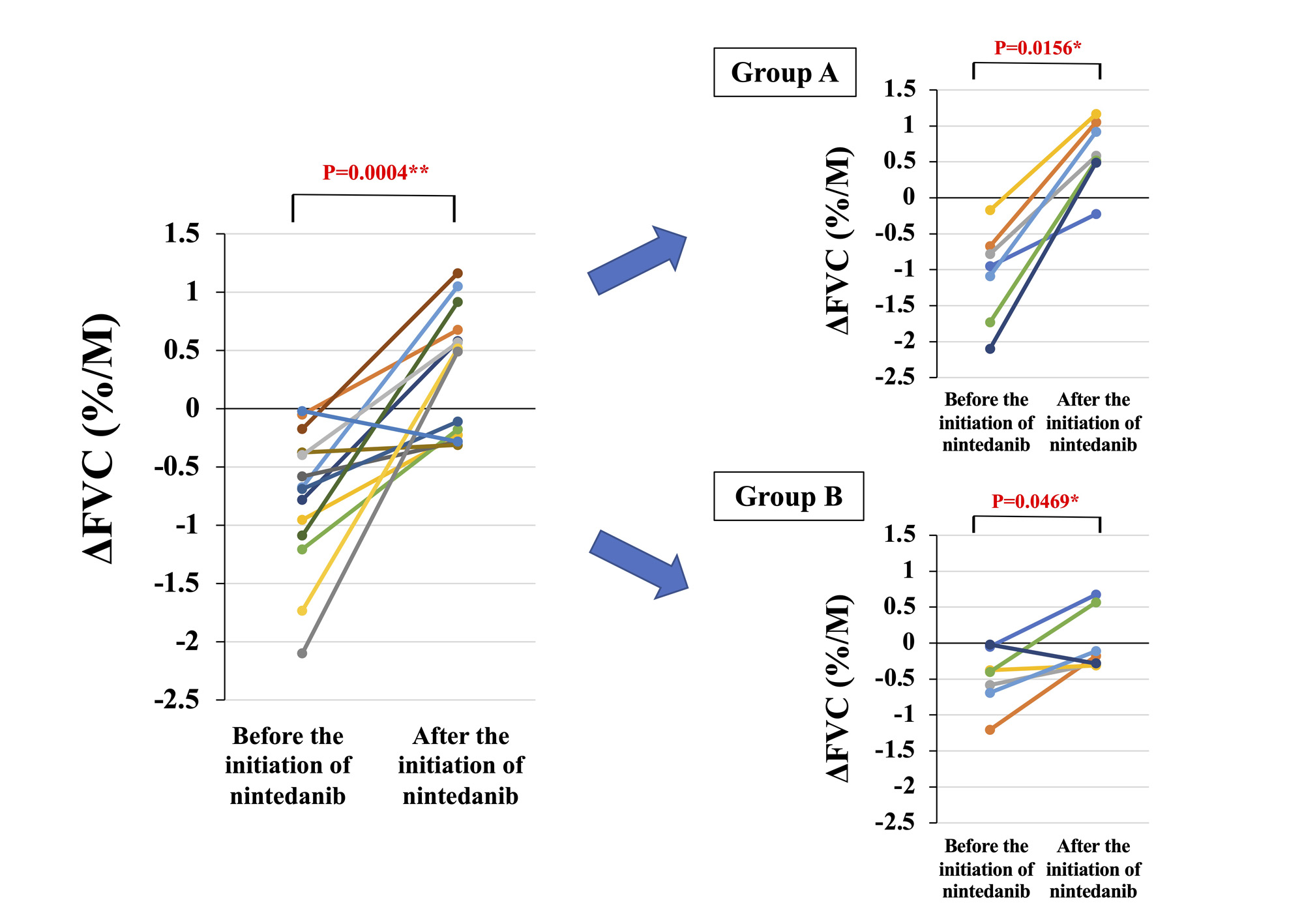
Results: Twenty-five patients with CTD-PF-ILD (8 males and 17 females) were included in the study. Patient characteristics are shown in Table 1. Among these CTDs complicated with PF-ILD, systemic sclerosis was the most common (9 cases; 36%), followed by myositis (6 cases; 24%). Mean FVC (%) increased from 59.1% to 65.3% and mean FVC (mL) increased from 1666.2 mL to 1821.4 mL at 6–12 months after administration of NTB (Figure 1). ΔFVC (%/M) significantly improved from -0.77%/M before NTB initiation to +0.33%/M after treatment (Figure 2). Serum KL-6 decreased significantly from 1461.6 U/mL to 1145.7 U/mL after 6–12 months of treatment. A significant negative correlation was found between the change of serum KL-6 and FVC (r: -0.7618, p: < .0001). Twelve of the 25 patients had new IS started after NTB initiation (Group A). Rituximab (6 patients) was the most commonly used as IS (Table 1). Twelve patients in Group A had a predominantly higher rate of improvement in ΔFVC (%) than the 13 patients in Group B (+12.1% vs. -0.41%, respectively) (Figure 1). The improvement in ΔFVC (%/M) was also greater in Group A than in Group B, but both groups had suppressed progressive pulmonary fibrosis (Figure 2). AEs considered to be treatment-related were diarrhea in 13 patients, nausea in 2 patients, elevated liver enzymes in 2 patients, and headache in 1 patient, respectively, and the dose of NTB was reduced in 11 patients. No patient discontinued NTB due to AEs.
Conclusion: NTB for CTD-PF-ILD appeared to be effective and safe in clinical practice. Our results also suggest that the combination of IS, including rituximab, may be more effective for CTD-PF-ILD than NTB treatment alone. Serum KL-6 may be a biomarker reflecting improvement in FVC during treatment with NTB.
To cite this abstract in AMA style:
Ushio Y, Wakiya R, Kameda T, Nakashima S, Shimada H, Miyagi T, Sugihara K, Mino R, Mizusaki M, Chujo K, Kagawa R, Yamaguchi H, Dobashi H. Nintedanib in Combination with Immunosuppressive Agents Improves Forced Vital Capacity in Connective Tissue Disease-associated PF-ILD: A Single-center Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/nintedanib-in-combination-with-immunosuppressive-agents-improves-forced-vital-capacity-in-connective-tissue-disease-associated-pf-ild-a-single-center-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nintedanib-in-combination-with-immunosuppressive-agents-improves-forced-vital-capacity-in-connective-tissue-disease-associated-pf-ild-a-single-center-study/