Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Interstitial lung disease (ILD) is a severe complication of autoimmune diseases (AD). In recent years, new strategies for the treatment of progressive pulmonary fibrosis (PPF) have been developed. Nintedanib (NINTE) has been the first antifibrotic drug approved for the treatment of progressive fibrosing autoimmune diseaserelated ILDs (INBUILD and SENSCIS trials).
The goals of our research were to evaluate the efficacy, safety and tolerance of NINTE in real-life AD-ILD patients in Spain.
Methods: A multicenter retrospective observational study was performed. We obtained data from medical records of 67 patients with AD-ILD treated with nintedanib 150 mg twice daily. Epidemiological and clinical data, highresolution computed tomography (HRCT) findings, pulmonary function tests (PFT), antibody status, and concomitant immunosuppressive drugs were also recorded. Statistical analyses were performed using Student’s test or Mann-Whitney’s T test for continuous variables and Chi2 test or Fisher’s exact test for categorical variables. A p value < 0.05 was considered significant.
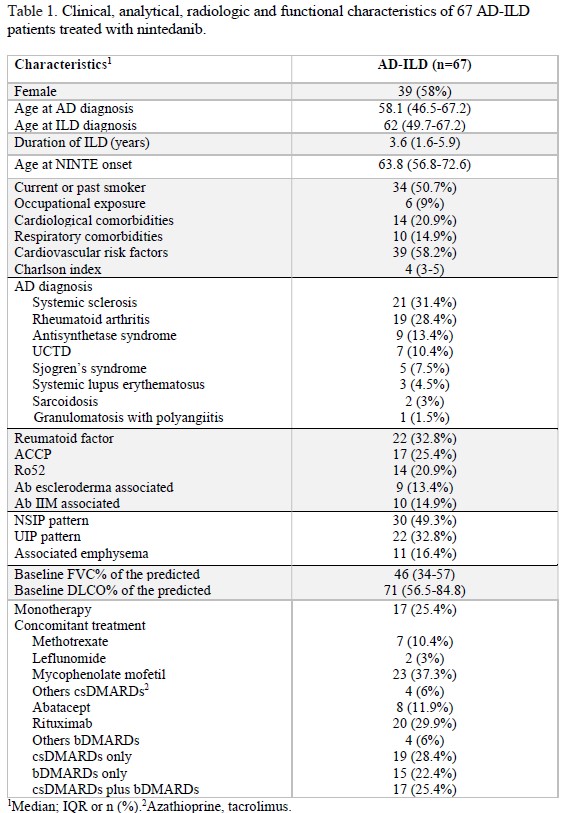
Results: 67 patients from six centers were included. 58% were female, median age at NINTE onset was 63.8 (56.8-72.6) years. The median disease duration was 4.1 (0.9-12.2) years for AD and 3.6 (1.6-5.9) years for ILD. Fourteen (23%) patients were diagnosed of rheumatic disease after ILD diagnosis. Clinical, immunological, radiological and treatment data are summarized in Table 1. NSIP pattern was associated with ANA (p=0.004) and cotreatment with mycophenolate mofetil (p=0.003) and rituximab (p=0.003). UIP pattern was associated with tobacco (p=0.046) and the presence of radiographic emphysema (p=0.032), RA patients (p=0.001), RF (p=0.001) and ACCP antibodies (p< 0.001). At one-year of follow-up, worsening of chest HRCT images was found in 31% of patients. Radiographic progression was associated with UIP pattern (OR 8.5 CI 1.5-46.8, p=0.014), RA diagnosis (OR 8, CI 51.7-38.4, p=0.017,) and ACCP antibodies (OR 11.2 CI 2.1-58.9, p=0.004). At baseline, predicted FVC% and DLCO% were 46 (34-57) and 71 (52.5-84.8); at six-month of follow-up were 46 (33-56) and 69 (57-82.5); and at one-year of follow-up were 44 (39.5-58) and 69 (54.8-84) respectively. No significant differences were found in PFT evolution between groups of HRCT patterns, rheumatic disease, autoantibodies and concomitant treatment. The median duration of NINTE treatment was 13.2 (8-26.9) months. Adverse events led to NINTE withdrawal in 13 patients, and 16 subjects required a dose reduction to 150 mg daily. During follow-up, 16
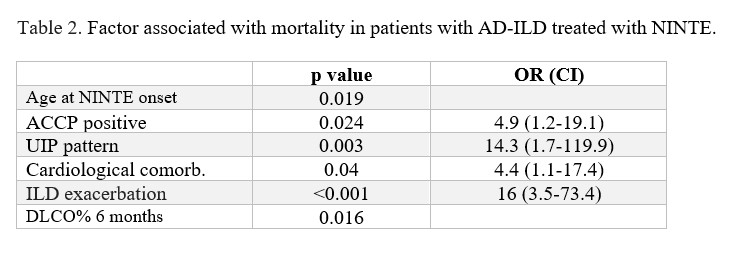
patients were admitted for ILD exacerbation, and 11 patients died. Ro52-positive patients and those treated with leflunomide were more likely to be admitted for pulmonary exacerbation. Factor associated with mortality are described in Table 2.
Conclusion: In our cohort, despite the great heterogeneity, NINTE appears to prevent interstitial lung progression in all subgroups regardless of the type of AD, concomitant treatment or time since diagnosis of pulmonary involvement. UIP pattern, ACCP antibodies, cardiological comorbidity, ILD exacerbation and DLCO% value at 6 months were linked to mortality.
To cite this abstract in AMA style:
pérez garcía p, Retuerto Guerrero M, chuquimia mendoza y, Loarce J, Atienza-Mateo B, Merino C, pavia pascual m, sieiro santos c, Moriano C, Diez álvarez e. Nintedanib in Autoimmune Disease-related Interstitial Lung Disease: Real-life Effectiveness, Safety and Tolerance in a Spanish Multicenter Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/nintedanib-in-autoimmune-disease-related-interstitial-lung-disease-real-life-effectiveness-safety-and-tolerance-in-a-spanish-multicenter-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nintedanib-in-autoimmune-disease-related-interstitial-lung-disease-real-life-effectiveness-safety-and-tolerance-in-a-spanish-multicenter-study/