Session Information
Date: Monday, November 14, 2022
Title: Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Gout flares result from an innate immune response against monosodium urate crystal deposits, resulting in macrophage crystal phagocytosis and cellular activation.1 NLRP3 inflammasome activation results in release of pro-inflammatory cytokines (e.g., IL-1b), attracting and stimulating bloodstream neutrophils, including triggering neutrophil extracellular traps (NETosis). The neutrophil-to-lymphocyte ratio (NLR) is a biomarker for systemic inflammation and is increased in patients with active polyarticular gout.2 However, little is known about the relationship between NLR and gout flare in patients undergoing urate-lowering therapy, or how treatment response influences NLR. We examined NLR changes in uncontrolled gout patients administered pegloticase as part of two pivotal trials.
Methods: Data from gout patients treated fortnightly with pegloticase (8 mg, 12 infusions) in two parallel phase 3 randomized trials were assessed by urate-lowering response (serum urate < 6 mg/dL for ≥80% of Months 3 and 6) and flare (upper flare tertile, ≥4 flares during pegloticase treatment) status. NLR was determined at baseline (BL, before pegloticase dose 1) and at Wks 7, 13, 19, and 25. Change from BL to Wk 25 was analyzed using a mixed model for repeated measures adjusting for age, visit, colchicine use (yes/no), group, and interaction between visit and group.
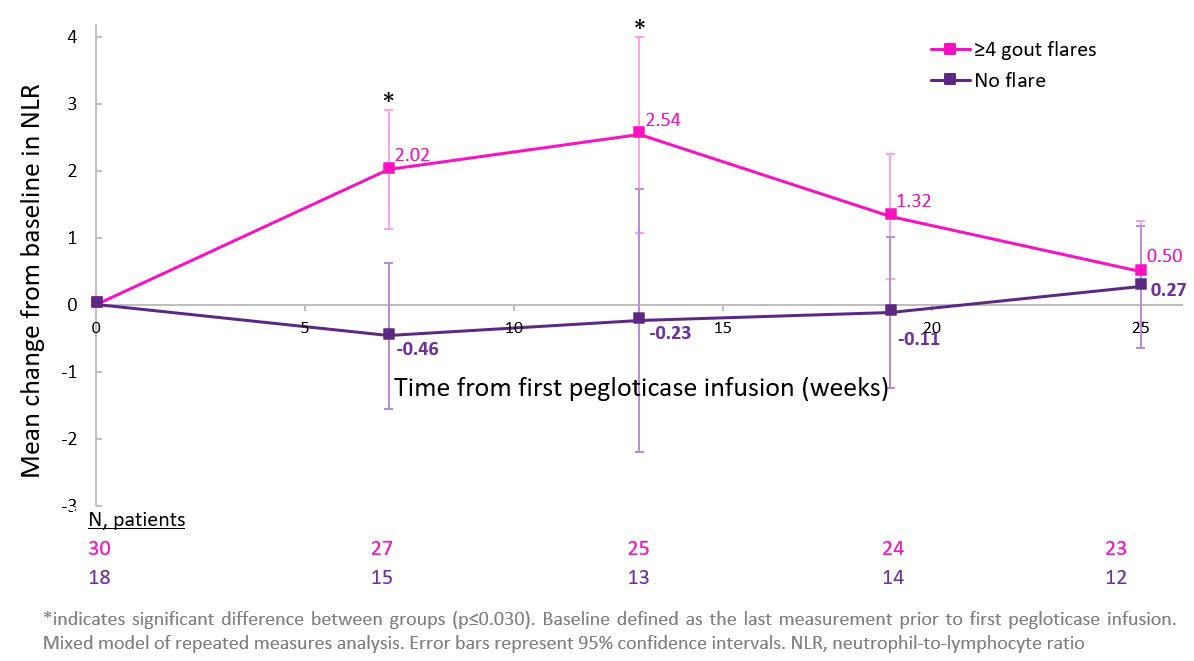
Results: 36 (72.2% men, 61.2±14.2 yrs) and 49 (85.7% men, 52.7±15.6 yrs) patients were pegloticase responders and non-responders, respectively. Mean (±SD) BL NLR was similar between responders (3.39±2.73) and non-responders (4.23±2.80, p=0.132) and near or above the normal NLR range (0.78─3.533). NLR peaked in responders (4.59±5.62) and non-responders (4.94±5.40) at Wk 13, returning to BL by Wk 25. Patients in the flare (3.75±2.38) and no flare (3.55±2.14) groups had similar BL NLR values. Median time to first flare was 9 days following first pegloticase infusion and flare rates progressively decreased following Month 1. Whereas peak mean (95%CI) change from BL in NLR was observed at Wk 13 in the flare group (+2.54 [+1.08, +4.00]; no meaningful change during treatment in the no flare group; Figure).
Conclusion: Sustained urate-lowering had little effect on NLR over the first 6 months of pegloticase therapy. However, flare patients had a significant NLR increase following therapy initiation, suggesting heightened systemic inflammation. Flare rates progressively decreased following treatment Month 1, but peak NLR occurred between Wks 13 and 19. Therefore, even though gout flares are generally thought of as local attacks, our findings suggest that heightened systemic inflammation persists for weeks to months following flare. Whether peak NLR at Wk 13 was a consequence of flare, or indicates a predisposition to flare and/or rising NLR remains unknown. Given that elevated NLR is associated with renal, cardiovascular, and overall mortality,4 further investigation in gout patients is warranted.
References
- So AK, Martinon F. Nat Rev Rheumatol 2017;13:639-47.
- Vedder D, et al. Arthritis Res Ther 2020;22:148.
- Forget P, et al. BMC Research Notes 2017;10:12.
- Song M, et al. Sci Rep 2021;11:464.
To cite this abstract in AMA style:
Pillinger M, Obermeyer K, Padnick-Silver L, LaMoreaux B. Neutrophil-to-lymphocyte Ratio Among Flaring and Non-flaring Uncontrolled Gout Patients Undergoing Pegloticase Therapy as Part of the Phase 3 Pivotal Trials [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/neutrophil-to-lymphocyte-ratio-among-flaring-and-non-flaring-uncontrolled-gout-patients-undergoing-pegloticase-therapy-as-part-of-the-phase-3-pivotal-trials/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neutrophil-to-lymphocyte-ratio-among-flaring-and-non-flaring-uncontrolled-gout-patients-undergoing-pegloticase-therapy-as-part-of-the-phase-3-pivotal-trials/