Session Information
Date: Tuesday, October 28, 2025
Title: (2052–2078) Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Dermatomyositis (DM) and anti-synthetase syndrome (AS) are idiopathic inflammatory myopathies (IIM) with perifascicular pathology involving vasculopathy, macrophages, dendritic cells and an overexpression of interferon-stimulated genes. Increased production and deficient elimination of neutrophil extracellular traps (NETs) are characteristic of the IIM, but their role as inducers of proinflammatory responses in the perifascicular pathology has not been addressed. The aim of this study was to assess the potential contribution of NETs to the perifascicular pathology in IIM.
Methods: We included 5 healthy donors (HD), 13 patients with DM and 12 with AS according to the ACR/EULAR 2017 and Connors’ criteria, either with moderate to severe disease activity or complete clinical response. We assessed the localization of macrophages and NETs in the muscle biopsies by confocal microscopy. From peripheral blood, we isolated normal density neutrophils to obtain spontaneous NETs and CD14+ monocytes for in vitro differentiation into macrophages and mDC for 7 days. Differentiated macrophages and myeloid dendritic derived cells from patients (PDM and PDmDC) and HD (HDM and HDmDC) were then stimulated for 6 hours with 50 µg of spontaneous NETs from patients and HD. Interferon signature was assessed by RT-qPCR differential gene expression of ISG15, IFI44, IFI44L, IFIT1, IFI27, CXCL9, RSAD2 (∆∆Ct equation) and IFN score was obtained by compound Z score.
Results: As shown in Figure 1, NETs are localized in key areas of perifascicular pathology, interacting with macrophages (A), inside muscle cells (B) and in the blood vessels (C). Stimulation with NETs significantly increased IFN-scores in patient and HD-derived macrophages and mDC compared to non-stimulated (Ns) cells (Figure 2 A and F). NETs isolated from IIM patients induced significantly stronger IFN responses than NETs from HD in both macrophages and mDCs (Figure 2B, 2G). Stratification by disease subtype revealed that NETs from DM patients elicited higher IFN-scores than those from AS patients in macrophages (p = 0.0354, Figure 2C), though this difference was not observed in mDCs (Figure 2H). No significant differences were detected based on disease activity (active vs inactive disease) in either cell type (Figure 2D, 2I). When analyzing combinations of cell type and NET origin, NETs from IIM patients consistently induced higher IFN responses across all cell types. In mDCs, IIM-derived NETs triggered significantly higher IFN-scores than HD-derived NETs in PDmDCs (p < 0.01, Figure 2J), while the difference in macrophages did not reach statistical significance (Figure 2E).
Conclusion: NETs from IIM patients trigger strong type I IFN responses in macrophages and mDC, particularly in cells derived from patients. This suggests that both, the composition of IIM NETs and the heightened response of in vivo primed myeloid cells contribute to the sustained IFN activity characteristic of IIM, pointing to a possible pathogenic loop of NETs driving chronic inflammation.
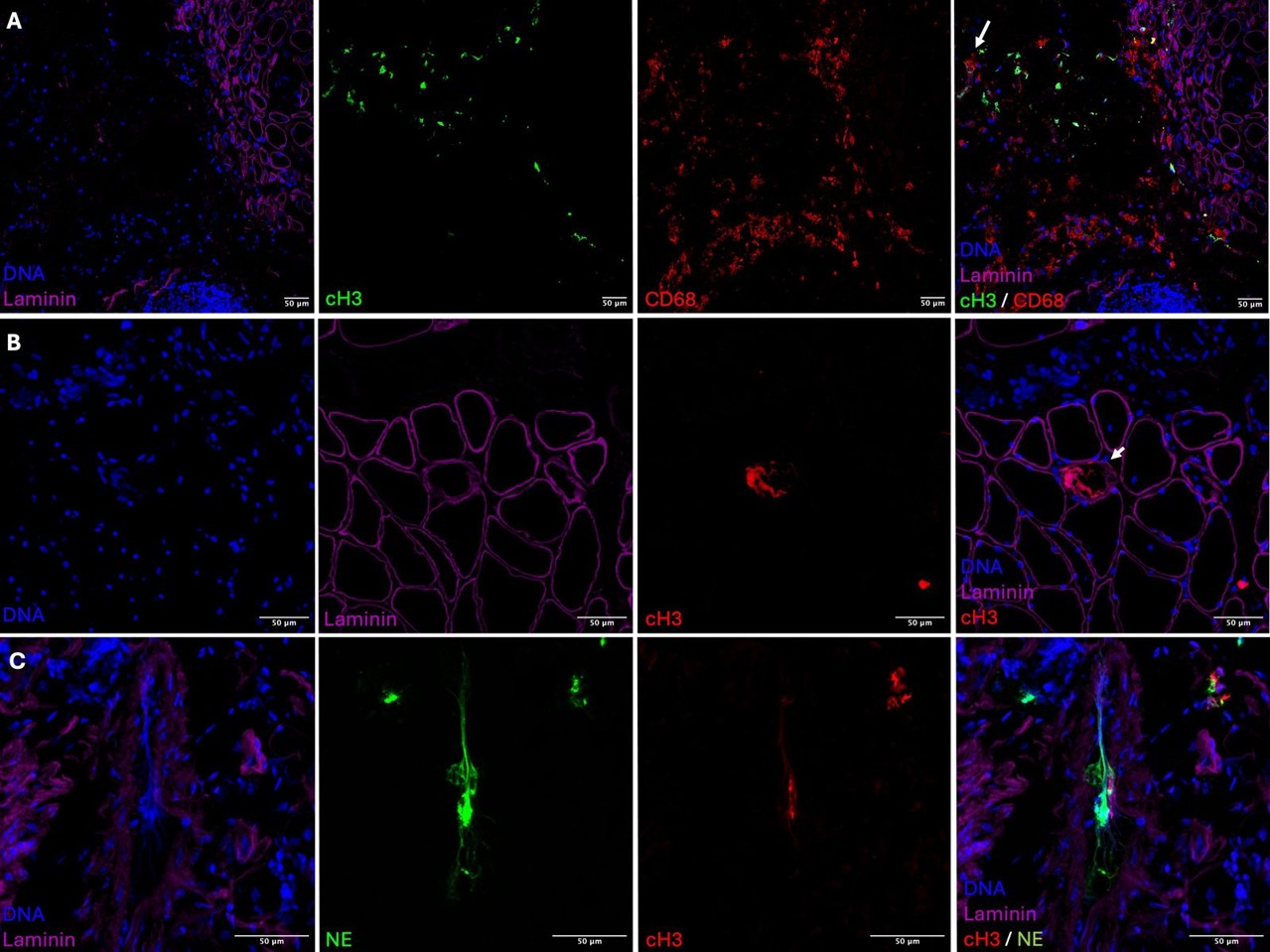 Figure 1. Localization of NETs in key areas of perifascicular pathology. Macrophages interact with NETs in the perifascicular area (A). NETs are internalized by muscle cells (B) and neutrophils produce NETs in the intravascular space (C).
Figure 1. Localization of NETs in key areas of perifascicular pathology. Macrophages interact with NETs in the perifascicular area (A). NETs are internalized by muscle cells (B) and neutrophils produce NETs in the intravascular space (C).
.jpg) Figure 2. In vitro evaluation of interferon score induction in macrophages and myeloid dendritic cells stimulated with NETs. Each figure represents an individual value. Bars represent the median and lines the interquartile range. Each figure represents an individual value. Bars represent the median and lines the interquartile range. (A) IFN-score in patients-derived macrophages (PDM) and healthy donor-derived macrophages (HDM), with or without NET stimulation (Ns). (B) IFN-score in HDM stimulated with NETs derived from IIM patients or healthy donors. (C) IFN-score in HDM stimulated with NETs from patients with dermatomyositis (DM) or antisynthetase syndrome (AS). (D) IFN-score in HDM stimulated with NETs from patients with active (aDM, aAS) or inactive (iDM, iAS) disease. (E) IFN-score in macrophages according to both cell origin (HDM vs PDM) and NET origin (HD vs IIM). (F) IFN-score in myeloid dendritic cells (mDCs) from patients (PDmDC) and healthy donors (HDmDC), with or without NET stimulation. (G) IFN-score in HDmDC and PDmDC stimulated with NETs from IIM patients or healthy donors. (H) IFN-score in mDCs stimulated with NETs from patients with DM or AS. (I) IFN-score in mDCs stimulated with NETs from patients with active (aDM, aAS) or inactive (iDM, iAS) disease. (J) IFN-score in mDCs based on both cell origin (HDmDC vs PDmDC) and NET origin (HD vs IIM). Mann-Whitney U test and Kruskal-Wallis test with Dunn post-hoc test; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Figure 2. In vitro evaluation of interferon score induction in macrophages and myeloid dendritic cells stimulated with NETs. Each figure represents an individual value. Bars represent the median and lines the interquartile range. Each figure represents an individual value. Bars represent the median and lines the interquartile range. (A) IFN-score in patients-derived macrophages (PDM) and healthy donor-derived macrophages (HDM), with or without NET stimulation (Ns). (B) IFN-score in HDM stimulated with NETs derived from IIM patients or healthy donors. (C) IFN-score in HDM stimulated with NETs from patients with dermatomyositis (DM) or antisynthetase syndrome (AS). (D) IFN-score in HDM stimulated with NETs from patients with active (aDM, aAS) or inactive (iDM, iAS) disease. (E) IFN-score in macrophages according to both cell origin (HDM vs PDM) and NET origin (HD vs IIM). (F) IFN-score in myeloid dendritic cells (mDCs) from patients (PDmDC) and healthy donors (HDmDC), with or without NET stimulation. (G) IFN-score in HDmDC and PDmDC stimulated with NETs from IIM patients or healthy donors. (H) IFN-score in mDCs stimulated with NETs from patients with DM or AS. (I) IFN-score in mDCs stimulated with NETs from patients with active (aDM, aAS) or inactive (iDM, iAS) disease. (J) IFN-score in mDCs based on both cell origin (HDmDC vs PDmDC) and NET origin (HD vs IIM). Mann-Whitney U test and Kruskal-Wallis test with Dunn post-hoc test; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
To cite this abstract in AMA style:
Reyna Juárez Y, Balderas Miranda J, Alcalá Carmona B, Ostos Prado M, santana k, Gómez-Martín D, Torres Ruiz J. Neutrophil Extracellular Traps from Patients with Idiopathic Inflammatory Myopathies Induce Interferogenic Responses in Macrophages and Myeloid Dendritic Cells [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/neutrophil-extracellular-traps-from-patients-with-idiopathic-inflammatory-myopathies-induce-interferogenic-responses-in-macrophages-and-myeloid-dendritic-cells/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neutrophil-extracellular-traps-from-patients-with-idiopathic-inflammatory-myopathies-induce-interferogenic-responses-in-macrophages-and-myeloid-dendritic-cells/
