Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Loss of bacterial diversity and increases of specific bacteria in the gut microbiota have been observed in inflammatory arthritis such as rheumatoid arthritis and spondyloarthritis. Alterations in the intestinal microbiota composition (dysbiosis) have also been found in experimental arthritis models, and the dysbiosis is involved in the susceptibility and severity of experimental arthritis. It is hypothesized that the dysbiosis-induced epithelial barrier disruption leads to latent inflammation in the lamina propria via increased intestinal permeability, and that the resulting alterations of intestinal immunity contribute to the development and progression of arthritis.
The gastrointestinal tract is innervated by the autonomic and sensory nervous systems, with nerve terminals reaching the lamina propria. Gut-innervating neurons release neurotransmitters and cytokines and regulate the gut microbiota through the control of in the production of cytokines from resident immune cells and antimicrobial peptides from intestinal epithelium. Therefore, gut-innervating neurons may be involved in the susceptibility and severity of arthritis via the gut microbiotal alterations.
Our aim is to determine the effect of enteric nervous system on arthritis via dysbiosis.
Methods: We utilized the Ncx/Hox11L.1 (Ncx) gene deficient (KO) and SKG (Th17-dependent arthritis model) mice. Ncx-KO mice have dysplasia of the intestinal plexus due to an increase in enteric neurons and exhibit dysbiosis and increased permeability of the intestinal mucosa due to excessive production of nerve-derived nitric oxide in the intestinal tract. Ncx-KO and SKG mice were mated to generate SKG/Ncx-KO mice. All mice were bred and manipulated under super-pathogen-free conditions, and arthritis was induced by intraperitoneal administration of mannan. Arthritis was evaluated by arthritis score, joint histopathology, numbers of inflammatory cells infiltrating the joint, and mRNA expression of cytokines in the joint tissue.
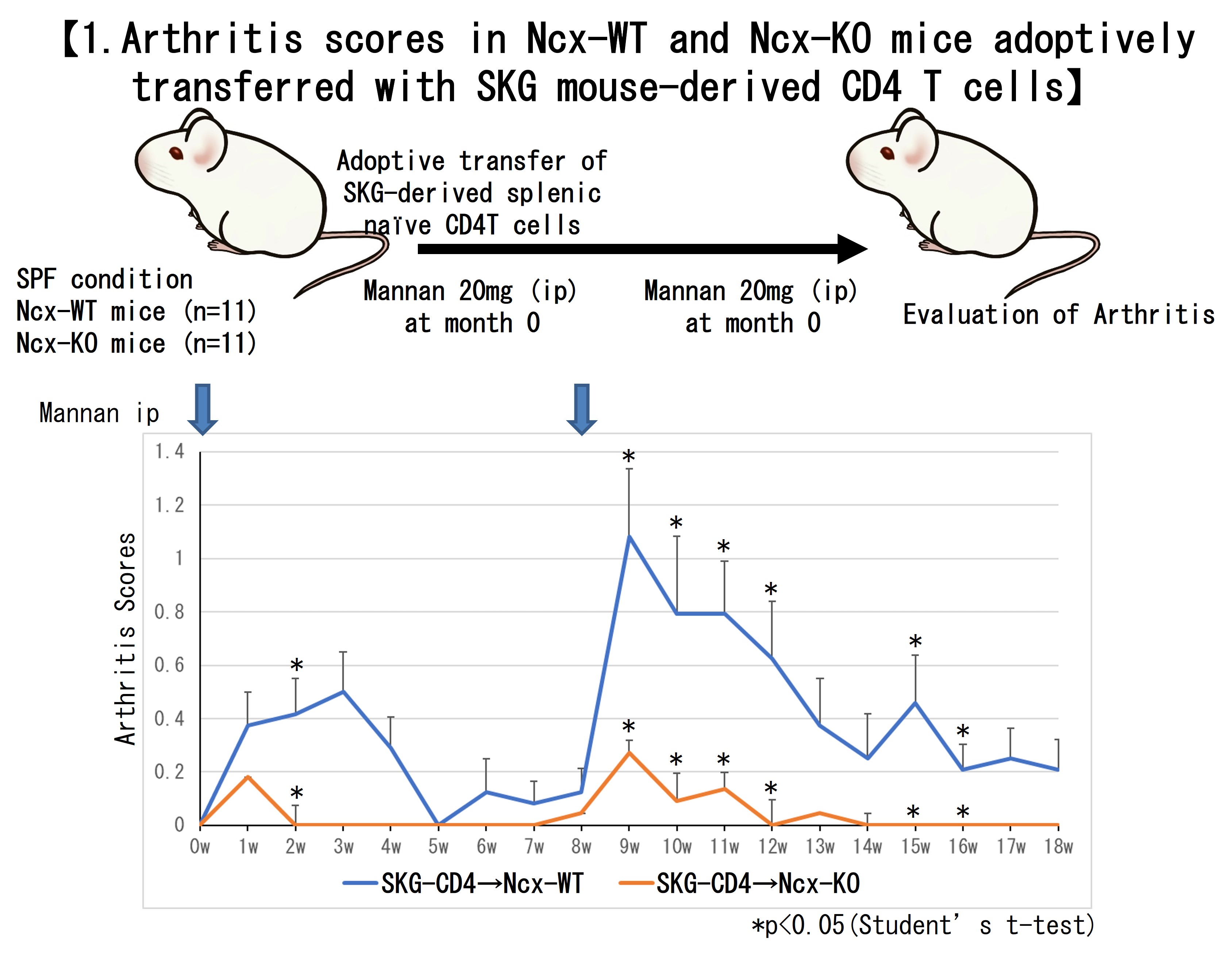
Results: First, SKG mouse-derived splenic naive CD4 T cells (SKG-CD4 T cells) were adoptively transferred into Ncx-KO and Ncx wild-type (WT) mice (n=7 each), respectively, and experimental arthritis was induced by mannan at week 0 and 8. Ncx-KO mice transplanted with SKG-CD4 T cells had significantly lower arthritis scores than Ncx-WT mice after the second mannan injection (p< 0.05) (Figure1).
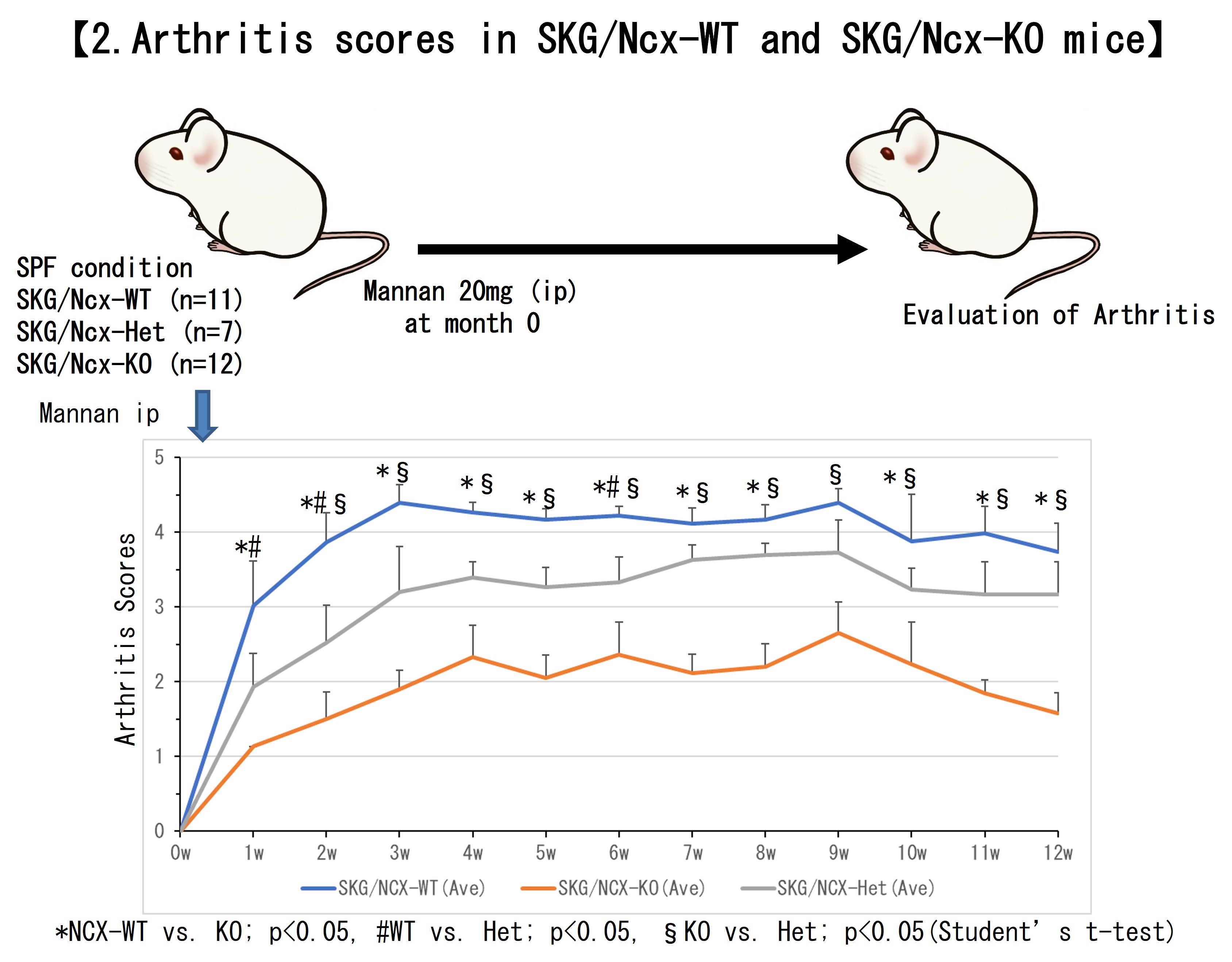
Next, the arthritis was induced in SKG/Ncx-KO and SKG/Ncx-WT mice. The scores of mannan-induced arthritis in SKG/Ncx-KO mice were reduced compared to SKG/Ncx-WT (n=11 each, p< 0.05) (Figure2). The joint histopathology scores, the number of inflammatory cells accumulating in joints, and cytokine production in joint tissue were lower in SKG/Ncx-KO mice than in SKG/Ncx-WT. In addition, the intestinal microbiota composition in SKG/Ncx-KO mice differed from that in SKG/Ncx-WT, and SKG/Ncx-KO mice had the gut microbiotal alterations.
Conclusion: Ncx-deficient enteric neuronal dysplasia reduced the severity of experimental arthritis with alterations in the composition of the gut microbiota. The enteric nervous system may influence the pathogenesis of inflammatory arthritis via the enteric microbiota.
To cite this abstract in AMA style:
Owada T, Hatanaka K, Tanaka A, Ikeda K, Hirata H, Fukushima Y, Kurasawa K, Arima M. Neuronal Intestinal Dysplasia Due to Ncx Gene Deficiency Attenuates the Severity of Experimental Arthritis in Mice [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/neuronal-intestinal-dysplasia-due-to-ncx-gene-deficiency-attenuates-the-severity-of-experimental-arthritis-in-mice/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/neuronal-intestinal-dysplasia-due-to-ncx-gene-deficiency-attenuates-the-severity-of-experimental-arthritis-in-mice/