Session Information
Date: Tuesday, October 28, 2025
Title: (1780–1808) Osteoarthritis & Joint Biology – Basic Science Poster
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: The neurotrophin, nerve growth factor (NGF), a key mediator of pain and inflammation, is increased in joints with osteoarthritis (OA). Neutralizing NGF with monoclonal antibodies has shown promising analgesic effects in painful knee OA, but clinical development was stopped owing to ill-understood side effects in the joints1. Knowledge about the biological effects of long-term exposure of healthy joint tissues to NGF is limited. Therefore, we aimed to explore the effects of repeated intra-articular (IA) injections of NGF into the knee joints of healthy mice on sensitization and pain, as well as joint innervation and structure.
Methods: All animal experiments were IACUC approved. Five experiments were conducted, all using 12-week old naïve male mice. In Experiment 1, NGF (50 ng or 500 ng in 5 uL, n=5 mice/group) or vehicle control (0.1% BSA in PBS, n=5 mice) was injected IA into the right knee joint of wildtype (WT) C57BL/6 mice, twice a week for 4 weeks. We assessed knee swelling and knee hyperalgesia as described2, as well as joint histopathology. Synovial changes including hyperplasia, cellularity and fibrosis were assessed by four different scorers blinded to treatment groups3. In Experiment 2, mice were injected with NGF (500ng) or vehicle, twice a week for 4 weeks and microCT of the knee was performed. In Experiment 3, NaV1.8-tdTomato reporter mice were injected with NGF (500ng) or vehicle, twice a week for 4 weeks, and joint innervation was assessed4. In Experiment 4, WT mice received 500ng NGF or vehicle twice a week for 4 weeks and were used for single cell RNA sequencing (scRNAseq) of the synovium. In Experiment 5, L3-L5 DRGs were collected from mice that received 3 IA injections of 500ng NGF or vehicle twice a week for 10 days and processed for bulk RNA sequencing.
Results: Repeated bi-weekly IA injections of both doses of NGF caused knee hyperalgesia in naïve mice (Fig. 1A). NGF caused dose-dependent knee swelling (Fig. 1B), synovial pathology (Fig. 1C), increased bone mineral density and trabecular bone thickness in the medial subchondral bone. NGF also caused osteophyte growth in the medial compartment, but no cartilage degeneration (Fig. 1D). NGF injection caused sprouting of NaV1.8+ neurons in the medial but not the lateral synovium (Fig. 1E). ScRNAseq of the synovium revealed upregulated genes related to neuronal sprouting, synovial fibrosis and ossification, confirming histopathological findings (Fig. 2). Bulk RNA seq of DRG showed upregulated pathways related to axonal growth (Fig. 3).
Conclusion: In healthy mouse knees, NGF induced mechanical sensitization, synovitis, neoinnervation in the medial synovium, and subchondral bone changes and osteophyte growth in the medial compartment, thus capturing many pathological changes observed in OA, except cartilage damage. It will be critical to describe in great depth the distribution of NGF and its receptors in all joint tissues, and how they change with age and disease. Such insights are especially important, as they may enable the modulation of NGF signaling to selectively target pain pathways while maintaining its protective functions. Hochberg, OAC, 2015. Miller, JCI Insight, 2018.Obeidat, OAC, 2024.Obeidat, OAC, 2019.
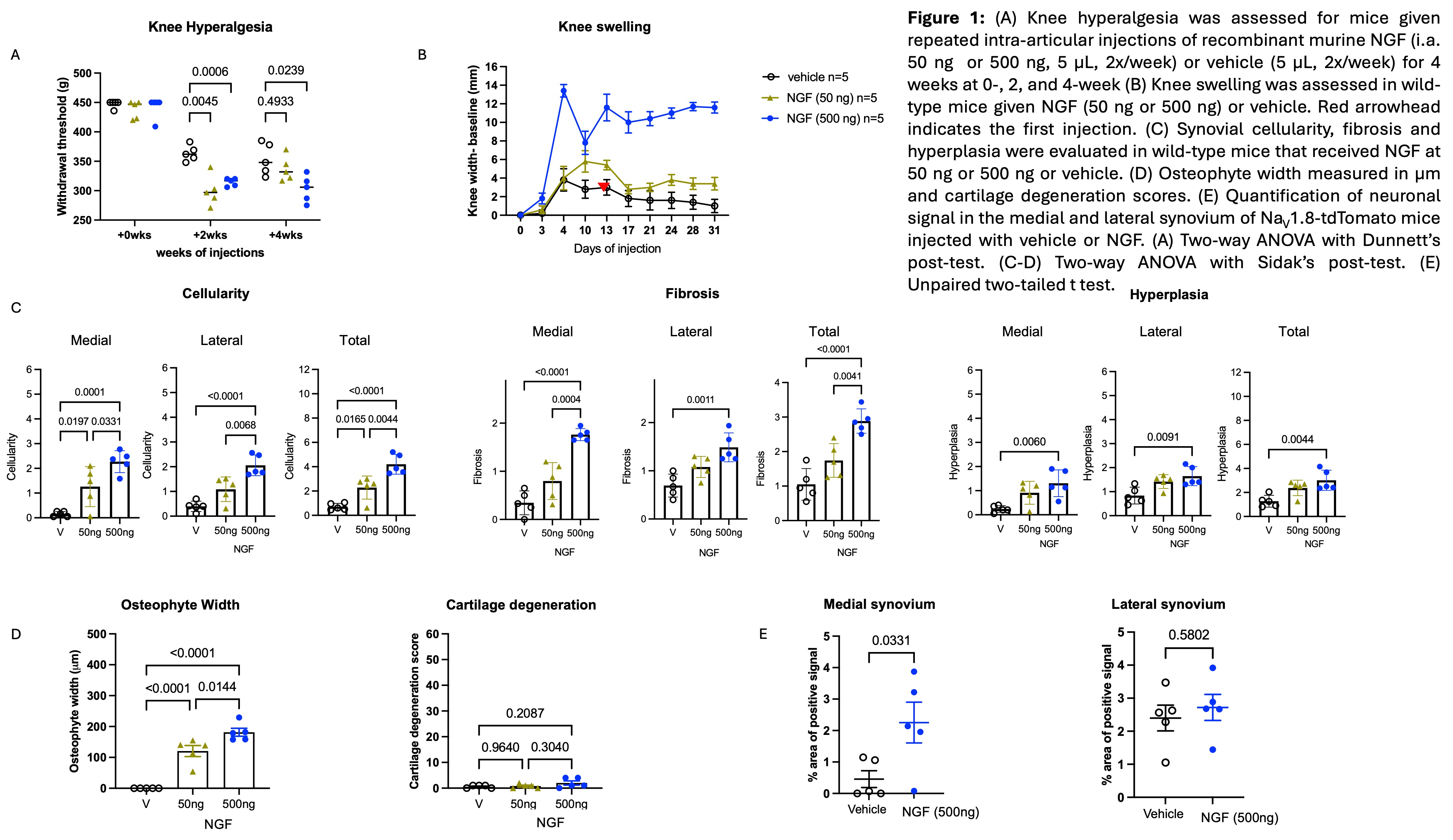 Figure 1: (A) Knee hyperalgesia was assessed for mice given repeated intra-articular injections of recombinant murine NGF (i.a. 50 ng or 500 ng, 5 µL, 2x/week) or vehicle (5 µL, 2x/week) for 4 weeks at 0-, 2, and 4-week (B) Knee swelling was assessed in wild-type mice given NGF (50 ng or 500 ng) or vehicle. Red arrowhead indicates the first injection. (C) Synovial cellularity, fibrosis and hyperplasia were evaluated in wild-type mice that received NGF at 50 ng or 500 ng or vehicle. (D) Osteophyte width measured in µm and cartilage degeneration scores. (E) Quantification of neuronal signal in the medial and lateral synovium of NaV1.8-tdTomato mice injected with vehicle or NGF. (A) Two-way ANOVA with Dunnett’s post-test. (C-D) Two-way ANOVA with Sidak’s post-test. (E) Unpaired two-tailed t test.
Figure 1: (A) Knee hyperalgesia was assessed for mice given repeated intra-articular injections of recombinant murine NGF (i.a. 50 ng or 500 ng, 5 µL, 2x/week) or vehicle (5 µL, 2x/week) for 4 weeks at 0-, 2, and 4-week (B) Knee swelling was assessed in wild-type mice given NGF (50 ng or 500 ng) or vehicle. Red arrowhead indicates the first injection. (C) Synovial cellularity, fibrosis and hyperplasia were evaluated in wild-type mice that received NGF at 50 ng or 500 ng or vehicle. (D) Osteophyte width measured in µm and cartilage degeneration scores. (E) Quantification of neuronal signal in the medial and lateral synovium of NaV1.8-tdTomato mice injected with vehicle or NGF. (A) Two-way ANOVA with Dunnett’s post-test. (C-D) Two-way ANOVA with Sidak’s post-test. (E) Unpaired two-tailed t test.
.jpg) Figure 2: NGF-induced changed in lining fibroblast transcriptome. (A) Heatmap of top gene set enrichment analysis-derived leading-edge genes, demonstrating coordinated upregulation of genes related to collagen fibril organization, smoothened signaling, and ossification. (B) Transcription factor binding motif analysis (RcisTarget) of lining fibroblasts in NGF v. Veh revealed significantly enriched motif transfac_pre_M01014 for the Sox family of transcription factors, predicted by direct annotation. (C) Expression of predicted transcription factors by lining fibroblasts in Veh and NGF groups, demonstrating highest expression of Sox5 (red arrow). (D) Gene regulatory network plot for Sox5 in lining fibroblasts.
Figure 2: NGF-induced changed in lining fibroblast transcriptome. (A) Heatmap of top gene set enrichment analysis-derived leading-edge genes, demonstrating coordinated upregulation of genes related to collagen fibril organization, smoothened signaling, and ossification. (B) Transcription factor binding motif analysis (RcisTarget) of lining fibroblasts in NGF v. Veh revealed significantly enriched motif transfac_pre_M01014 for the Sox family of transcription factors, predicted by direct annotation. (C) Expression of predicted transcription factors by lining fibroblasts in Veh and NGF groups, demonstrating highest expression of Sox5 (red arrow). (D) Gene regulatory network plot for Sox5 in lining fibroblasts.
.jpg) Figure 3: Differential gene expression analysis on bulk RNAseq conducted on DRGs collected after 3 injections of NGF or vehicle. (A) Volcano plot showing all genes that were not filtered out by DESeq2 (15,664). Red indicates genes that met the Padj cut-off < 0.05. No fold change cutoff was applied. Genes of interest are labeled. (B) Top 50 genes by padj, ordered by log2 fold change with the size of the bubble mapped to the adjusted p-value. String network showing known relationships to Ngf and to Ntrk1 (highlighted in yellow). (C) Functional enrichment was performed on the upregulated DEG list using Cytoscape String Enrichment; Interacting genes contributing to the top 5 Reactome pathways are shown using a redundancy cutoff of 0.5. Hub genes are highlighted in yellow.
Figure 3: Differential gene expression analysis on bulk RNAseq conducted on DRGs collected after 3 injections of NGF or vehicle. (A) Volcano plot showing all genes that were not filtered out by DESeq2 (15,664). Red indicates genes that met the Padj cut-off < 0.05. No fold change cutoff was applied. Genes of interest are labeled. (B) Top 50 genes by padj, ordered by log2 fold change with the size of the bubble mapped to the adjusted p-value. String network showing known relationships to Ngf and to Ntrk1 (highlighted in yellow). (C) Functional enrichment was performed on the upregulated DEG list using Cytoscape String Enrichment; Interacting genes contributing to the top 5 Reactome pathways are shown using a redundancy cutoff of 0.5. Hub genes are highlighted in yellow.
To cite this abstract in AMA style:
obeidat a, Newton m, Li J, Hu B, Ishihara S, Lammlin L, Junginger L, Farrell E, Ko F, Miller R, Scanzello C, Maerz T, Miller R, Malfait A. Nerve Growth Factor Causes Sensitization, Nociceptor Sprouting, Synovitis, And Osteophyte Formation In Naïve Murine Joints [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/nerve-growth-factor-causes-sensitization-nociceptor-sprouting-synovitis-and-osteophyte-formation-in-naive-murine-joints/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nerve-growth-factor-causes-sensitization-nociceptor-sprouting-synovitis-and-osteophyte-formation-in-naive-murine-joints/
