Session Information
Session Type: Abstract Session
Session Time: 8:00AM-9:00AM
Background/Purpose: Guidelines recommend testing for latent hepatitis B virus (HBV), hepatitis C virus (HCV), and tuberculosis (TB) infection prior to initiating biologics or targeted synthetic disease modifying anti-rheumatic drugs (b/tsDMARDs) to avoid reactivation of these life-threatening infections. Despite these recommendations, previous studies have found that a minority of patients in U.S. rheumatology practices have documented testing for all latent infections. We developed and tested a web-based dashboard for clinicians within the Veterans Health Administration (VHA) with the goal of improving the safe prescribing of b/tsDMARDs.
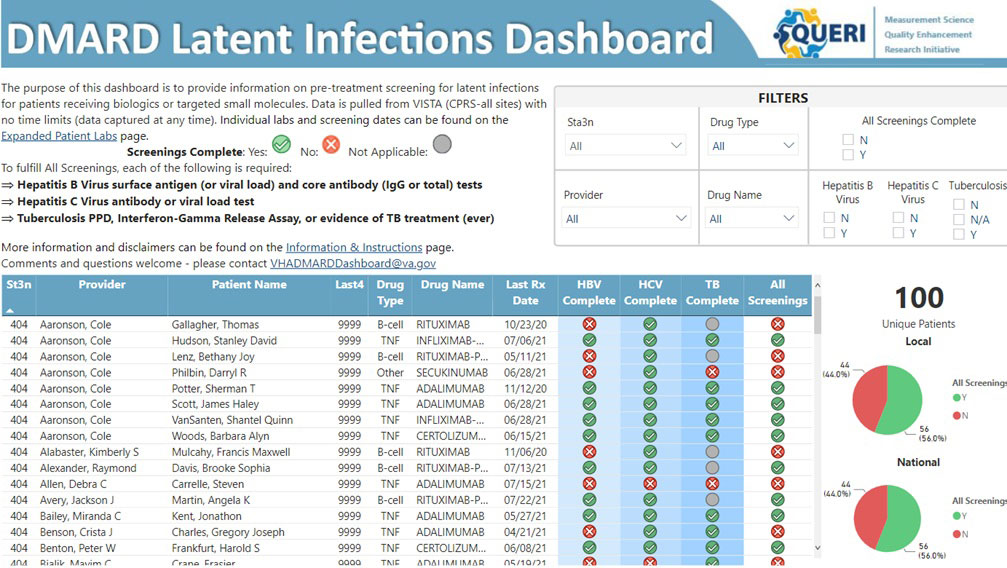
Methods: The dashboard was created using Power BI (Microsoft), a data management software package, and launched on 1/11/2022. Data displayed on the dashboard are extracted from the VHA corporate data warehouse and includes patient identifiers, prescribed b/tsDMARDs, most recent prescription date, dates of testing for HBV (surface antigen (or viral load) AND core antibody); HCV (antibody or viral load); and TB (PPD, Interferon-Gamma Release Assay, or evidence of treatment). National and facility-level performance (percent of patients with complete testing) are shown for benchmarking (Figure 1). Clinicians can access the dashboard via the VHA intranet and use the information to enter missing test orders directly into the EHR.
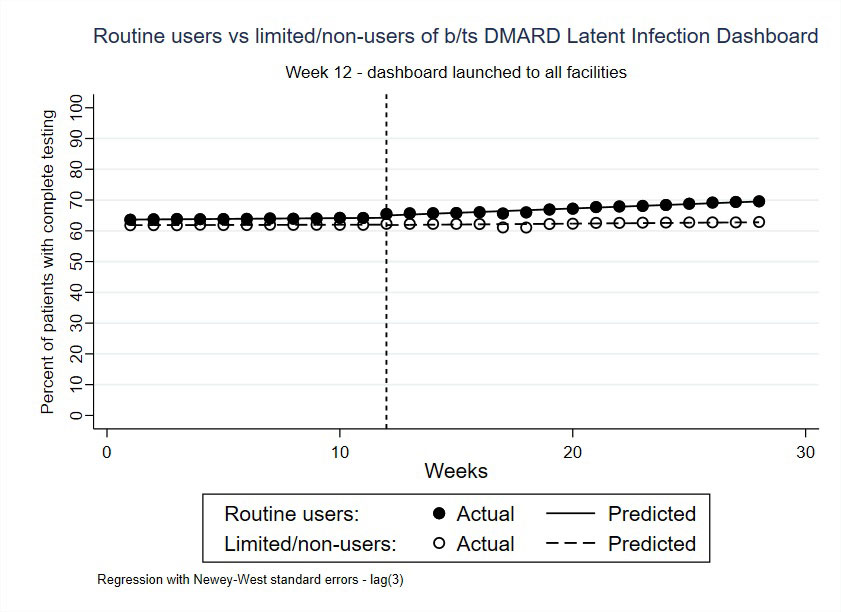
We performed an interrupted time-series (ITS) analysis to assess whether use of the dashboard affected the percent of patients with complete testing across 129 VHA facilities. Facilities were divided into 2 groups according to the total of dashboard logins from that facility (above/below the median, those with > 8 logins were designated “routine users” vs. ≤ 8 logins “limited/non-users”). The percent of patients with complete testing was assessed weekly between 11/1/2021 – 1/10/2022 (prior to dashboard launch, “baseline period”) and 1/11/2022 – 5/9/2022 (after dashboard launch, “intervention period”). We compared routine vs. limited/non-users using an ITS model which adjusted for facility-level characteristics.
Results: There were 50,176 Veterans prescribed b/tsDMARDs nationwide. 31 facilities qualified as routine users and 98 were limited/non-users. Across all sites at baseline, 62.7% of patients had complete testing. The percent of patients with complete testing improved for routine users during the study period (63.6% to 69.6%), whereas limited/non-users remained stable (61.9% to 62.9%). The post-intervention linear trend for routine vs. limited/non-users was 0.23, 95% CI (0.17-0.28, p = 0.01; Figure 2), representing a small but significant improvement for routine users over this 7-month period.
Conclusion: Use of a medication safety dashboard designed to support the safe prescribing of b/tsDMARDs resulted in small but significant improvements among facilities who used the dashboard routinely compared to those with fewer logins. We anticipate further improvements over time and as additional facilities achieve routine use. Rapid deployment of EHR-based dashboards can facilitate quality improvement by providing actionable reports directly to clinicians.
To cite this abstract in AMA style:
Montgomery A, Tarasovsky G, Whooley M, Barton J, Miller K, Mitchell H, Dana J, Reiter K, Wahl E, Rozenberg-Ben-Dror K, Li J, Schmajuk G. National Rollout of a Medication Safety Dashboard to Improve Testing for Latent Infections Among Biologic/targeted Synthetic DMARD Users Within the Veterans Health Administration: Initial Results [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/national-rollout-of-a-medication-safety-dashboard-to-improve-testing-for-latent-infections-among-biologic-targeted-synthetic-dmard-users-within-the-veterans-health-administration-initial-results/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/national-rollout-of-a-medication-safety-dashboard-to-improve-testing-for-latent-infections-among-biologic-targeted-synthetic-dmard-users-within-the-veterans-health-administration-initial-results/