Session Information
Date: Tuesday, October 28, 2025
Title: Abstracts: Metabolic & Crystal Arthropathies – Basic & Clinical Science (2585–2590)
Session Type: Abstract Session
Session Time: 1:30PM-1:45PM
Background/Purpose: Patients (pts) with uncontrolled gout (UG; persistent elevation in serum uric acid [sUA] levels and clinical manifestations despite oral urate-lowering therapy) have poor health-related quality-of-life (HRQOL) outcomes. The global UG disease burden is substantial and increasing. NASP (formerly SEL-212) is an investigational, novel, every 4-wk (Q4W), sequential 2-component infusion therapy consisting of targeted nanoencapsulated sirolimus (NAS, formerly SEL-110), which mitigates the formation of antipegadricase antibodies, and a uricase (pegadricase [P]; formerly SEL-037), which reduces sUA. Here, we report sUA levels, joint exam findings, and HRQOL outcomes in pts with UG who received 6 doses of NASP or placebo (PBO) in the phase 3 DISSOLVE I and II (NCT04513366, NCT04596540) studies.
Methods: Eligible pts had a history of symptomatic gout (≥3 gout flares within 18 mo of screening, ≥1 tophus, or current gouty arthritis), inability to normalize sUA and control symptoms with treatment, and screening sUA ≥7 mg/dL, which aligned with ACR diagnostic criteria for gout (Neogi et al. Arthritis Rheumatol 2015). Pts were randomized 1:1:1 to receive high-dose (HD) NASP (0.15 mg/kg NAS; 0.2 mg/kg P), low-dose (LD) NASP (0.10 mg/kg NAS; 0.2 mg/kg P), or PBO Q4W; those who received 6 doses were included in this post hoc analysis. Pt-reported outcomes (PROs) included the 36-Item Short Form Health Survey (SF-36) physical component summary (PCS) score (minimal clinically important difference [MCID], 5) and Health Assessment Questionnaire-Disability Index (HAQ-DI) pain score (MCID, 16).
Results: In pts receiving HD NASP (n=42), LD NASP (n=35), or PBO (n=67), mean sUA changes of −88%, −94%, and −0.3% (median: −97%, −98%, and 1.5%) were observed from baseline (mean [standard deviation (SD)]: 8.5 [1.4], 8.5 [1.3], and 8.7 [1.6] mg/dL) to wk 24 (mean [SD]: 1.1 [2.0], 0.5 [0.8], and 8.5 [2.0] mg/dL), respectively. In the NASP arms, sUA reduction was noted immediately after the first dose, and mean sUA generally remained ≤2.0 mg/dL throughout the study (Figure 1A). Pts receiving HD NASP, LD NASP, and PBO had mean reductions in the number of tender (−74%, −92%, and −40%) and swollen (−90%, −91%, and −54%) joints from baseline (mean number of tender/swollen joints: 5.8/2.9, 6.1/3.5, and 7.7/5.2) through wk 24 (mean number of tender/swollen joints: 1.5/0.3, 0.5/0.3, and 4.6/2.4). HD and LD NASP generally led to an approximately 2-fold reduction in the mean number of tender and swollen joints vs PBO (Figure 1B). NASP-treated pts showed improvement in mean SF-36 PCS scores (HD NASP: 5.9; LD NASP: 9.7) that exceeded the MCID and was >2-fold higher than the change for PBO-treated pts by wk 24 (2.8); NASP-treated mean SF-36 PCS scores (HD NASP: 45.2; LD NASP: 46.4) were comparable to the US general population (49.2; Maglinte et al. J Clin Epidemiol 2012). By wk 24, a greater mean HAQ-DI pain score reduction was seen with NASP (HD NASP: −60%; LD NASP: −80%) vs PBO (−44%; Figure 2).
Conclusion: NASP-treated pts had meaningful reductions in disease burden and key clinical manifestations, as reflected by improvements in sUA levels and PROs. This therapy has a noteworthy impact on resolving acute gout symptoms over 6 treatments and shows promise for improving HRQOL.
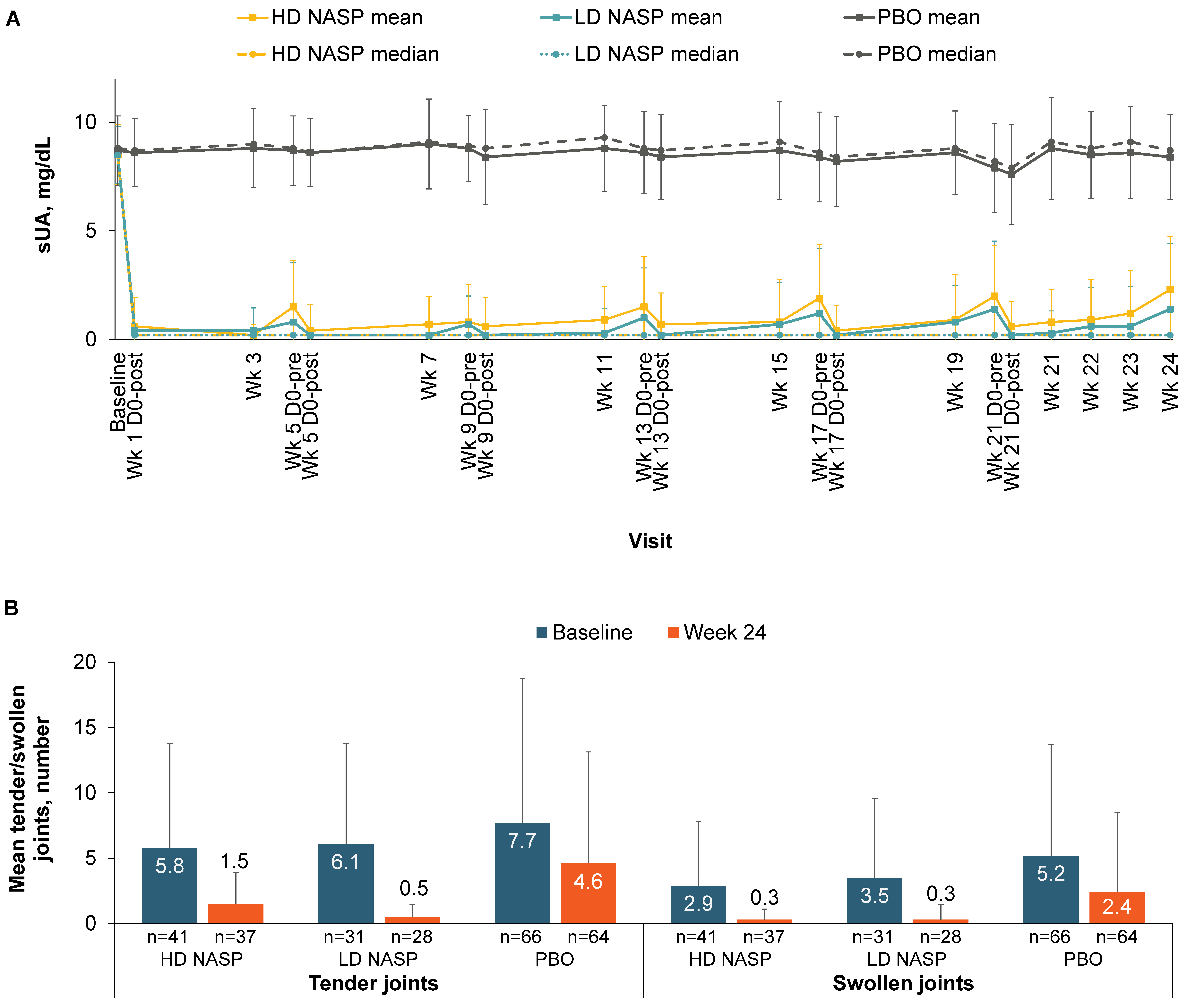 Figure 1: (A) sUA Over Time and (B) Number of Tender/Swollen Joints at Baseline and Week 24. Error bars show standard deviation for both panels. In panel A, n values were 42 at baseline and 37 at week 24 for HD NASP, 35 at both time points for LD NASP, and 67 at both time points for PBO.
Figure 1: (A) sUA Over Time and (B) Number of Tender/Swollen Joints at Baseline and Week 24. Error bars show standard deviation for both panels. In panel A, n values were 42 at baseline and 37 at week 24 for HD NASP, 35 at both time points for LD NASP, and 67 at both time points for PBO.
D, day; HD NASP, high-dose NASP; LD NASP, low-dose NASP; n, number of patients; NASP, nanoencapsulated sirolimus plus pegadricase; PBO, placebo; post, post-dose; pre, pre-dose; sUA, serum uric acid; Wk, week.
.jpg) Figure 2: SF-36 PCS Score and HAQ-DI Pain Score at Baseline and Week 24. Error bars show standard deviation. Higher SF-36 scores indicate greater improvement in health-related quality of life; lower HAQ-DI scores indicate greater improvement in functional ability and less disability.
Figure 2: SF-36 PCS Score and HAQ-DI Pain Score at Baseline and Week 24. Error bars show standard deviation. Higher SF-36 scores indicate greater improvement in health-related quality of life; lower HAQ-DI scores indicate greater improvement in functional ability and less disability.
HAQ-DI, Health Assessment Questionnaire-Disability Index; HD NASP, high-dose NASP; LD NASP, low-dose NASP; n, number of patients; NASP, nanoencapsulated sirolimus plus pegadricase; PBO, placebo; PCS, physical component summary; SF-36, 36-Item Short Form Health Survey.
To cite this abstract in AMA style:
Khanna P, Majjhoo A, Azeem R, Peace B, Desai B, Strand V. Nanoencapsulated Sirolimus Plus Pegadricase Reduced Disease Burden in Patients With Uncontrolled Gout: Results From the Phase 3 DISSOLVE Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/nanoencapsulated-sirolimus-plus-pegadricase-reduced-disease-burden-in-patients-with-uncontrolled-gout-results-from-the-phase-3-dissolve-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/nanoencapsulated-sirolimus-plus-pegadricase-reduced-disease-burden-in-patients-with-uncontrolled-gout-results-from-the-phase-3-dissolve-trials/
