Session Information
Date: Monday, November 13, 2023
Title: Abstracts: Pediatric Rheumatology – Clinical II: Connective Tissue Disease
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
Background/Purpose: Myositis-associated autoantibodies (MAAs), such as anti-Ro52 autoantibodies (Abs), have been found to be associated with interstitial lung disease (ILD) and worse prognosis in the idiopathic inflammatory myopathies. MAAs remain largely uncharacterized in juvenile-onset myositis. Moreover, MAAs often co-exist and it is unknown whether the number of MAA specificities may be associated with increased disease severity.
Methods: Patients with juvenile myositis enrolled in cross-sectional NIH myositis natural history studies who underwent testing for myositis Abs were included. Demographics, clinical manifestations, treatments, and outcomes of those with and without MAAs were compared using Chi-squared, Fisher’s exact test, or Wilcoxon rank-sum test. Multivariable logistic regression with adjustment for year of diagnosis and myositis autoantibodies was performed for statistically significant variables from the univariable analyses. Multivariable logistic regression was also used to determine whether the number of MAA specificities is predictive of severe disease features. A two-sided p< 0.05 was considered significant.
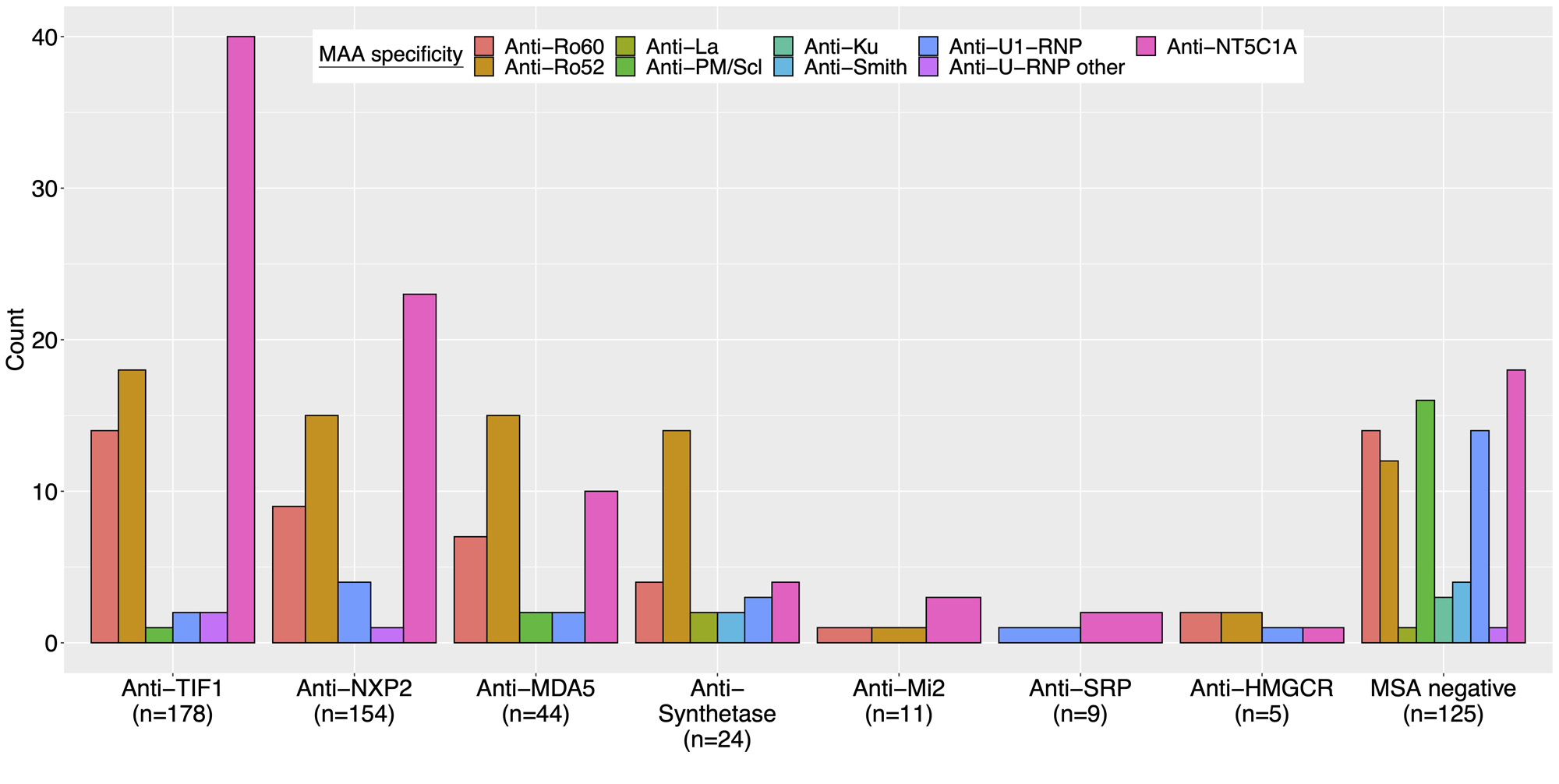
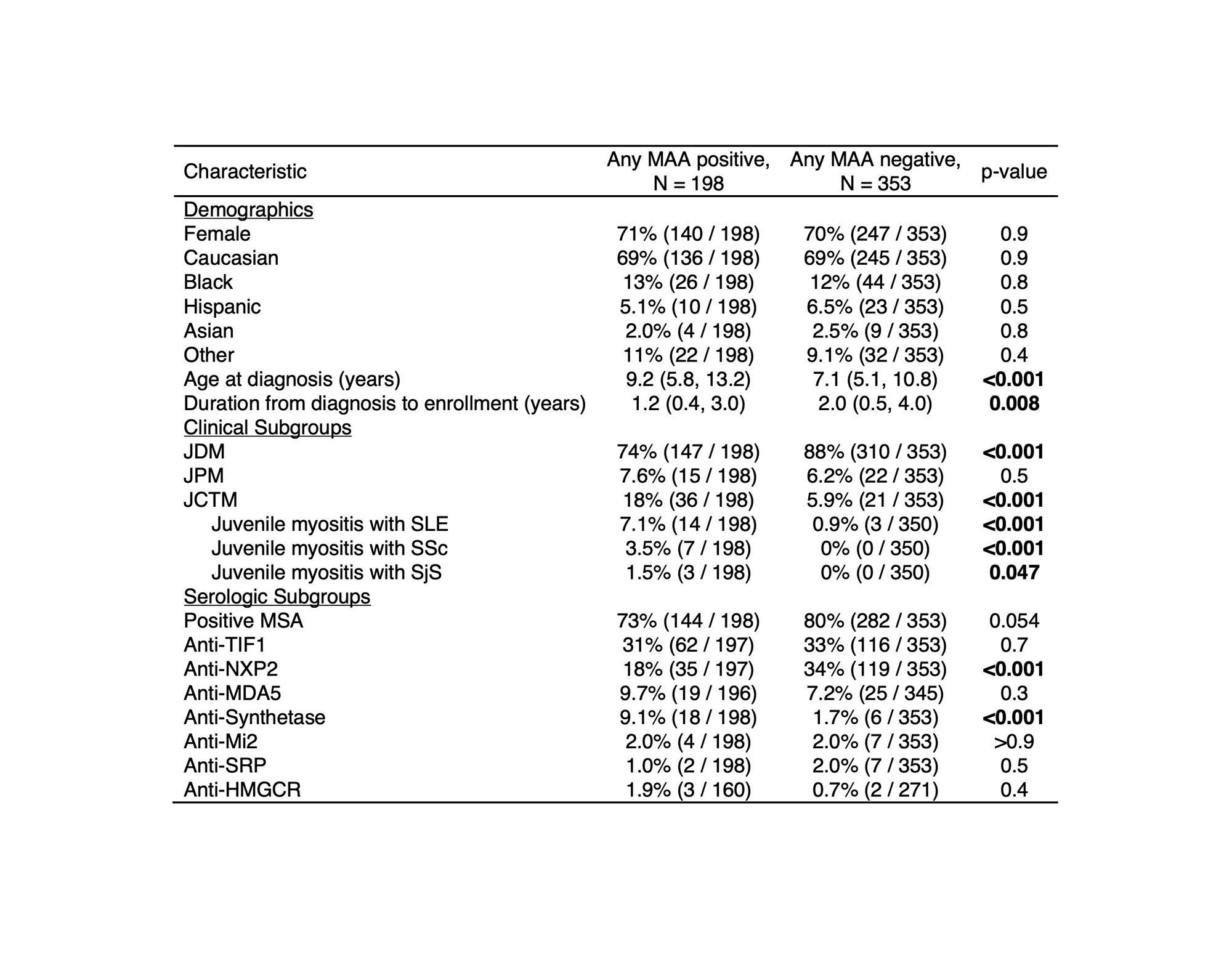
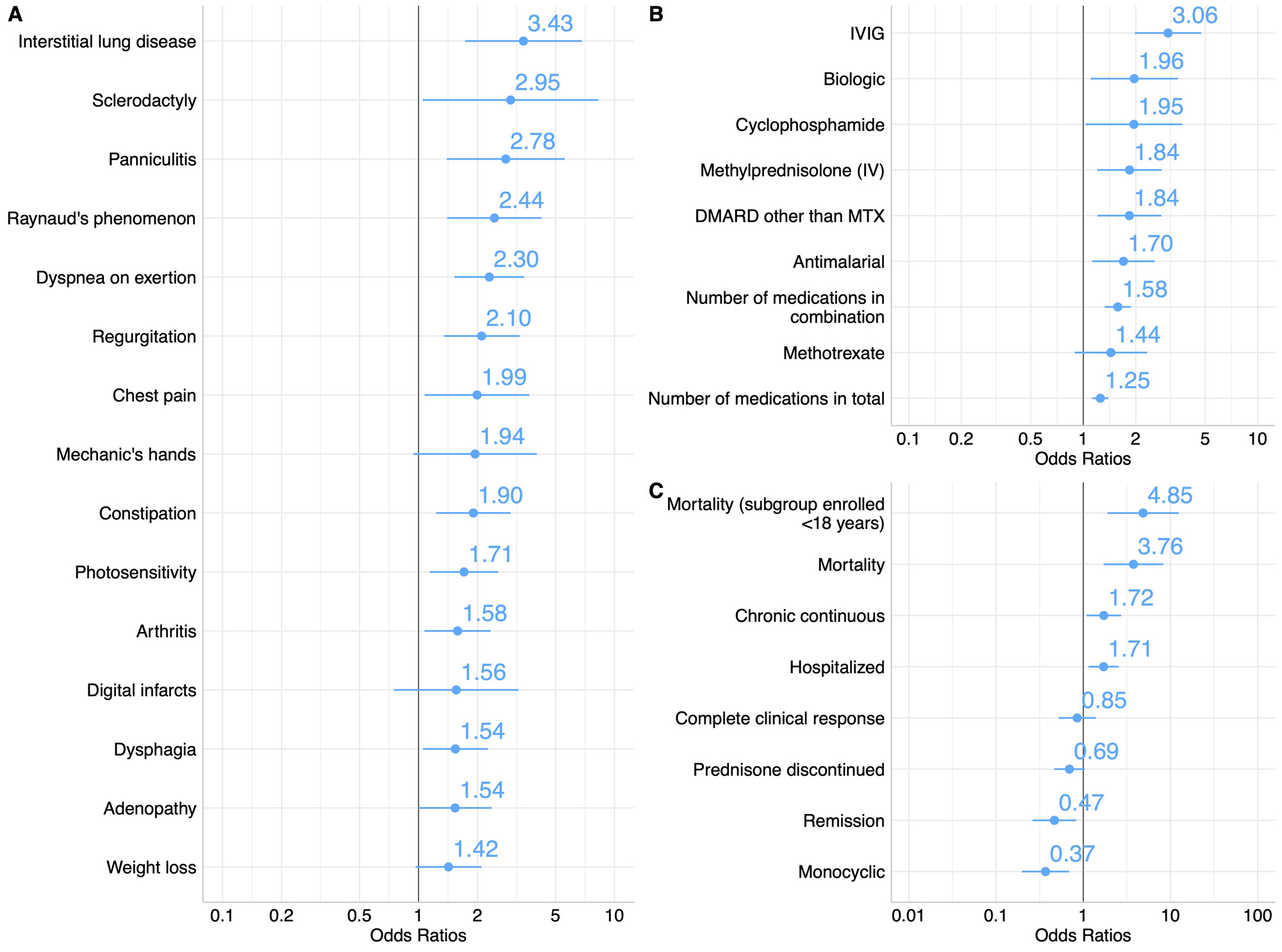
Results: MAAs were present in 36% of this North American cohort of 551 juvenile myositis patients and were found across serologic subgroups (Figure 1). Among patients with any MAA, there was a higher frequency of juvenile connective tissue myositis (JCTM) (18% vs 5.9%, p< 0.001) and lower frequency of juvenile dermatomyositis (JDM) (74% vs 88%, p< 0.001) (Table 1). Anti-synthetase Abs (9.1% vs 1.7%, p< 0.001) were more frequent and anti-NXP2 Abs (18% vs 34%, p< 0.001) less frequent among those with an MAA. The presence of an MAA was associated with certain clinical manifestations, such as ILD (OR 3.43; 95% CI 1.75-6.96), Raynaud’s phenomenon (OR 2.44; 95% CI 1.41-4.28), and arthritis (OR 1.58; 95% CI 1.07-2.34) (Figure 2). The presence of any MAA was associated with increased treatment burden, including a greater odds of receiving IVIG (OR 3.06; 95% CI 1.98-4.76), IV methylprednisolone (OR 1.84; 95% CI 1.21-2.83), and a greater number of major medications in combination (OR 1.58; 95% CI 1.33-1.88). Positivity for any MAA was also associated with worse outcomes, including higher odds of mortality (OR 3.76; 95% CI 1.72-8.43) and a chronic continuous disease course (OR 1.72; 95% CI 1.10-2.72) and lower odds of a monocyclic disease course (OR 0.37; 95% CI 0.19-0.67) and achievement of remission (OR 0.47; 95% CI 0.26-0.82). Moreover, the number of MAA specificities was predictive of mortality (OR 1.83; 95% CI 1.16-2.86).
Conclusion: MAAs were prevalent in this large cohort of patients with juvenile myositis and were associated with severe disease features, more refractory disease, and mortality. Future prospective studies are needed to determine whether early detection of MAAs may improve outcomes for patients with juvenile myositis.
Acknowledgements: Intramural Research Program of NIAMS and NIEHS of the NIH.
Abbreviations: JCTM, juvenile connective tissue myositis; JDM, juvenile dermatomyositis; JPM, juvenile polymyositis; MAA, myositis-associated autoantibody; MSA, myositis-specific autoantibody; SjS, Sjogren’s syndrome; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
Abbreviations: DMARD, disease-modifying antirheumatic drug; IV, intravenous; IVIG, intravenous immunoglobulin; MTX, methotrexate.
To cite this abstract in AMA style:
Sherman M, Noroozi Farhad P, Pak K, Pinal-Fernandez I, Sarkar K, Neely M, Targoff I, Miller F, Mammen A, Rider L. Myositis-associated Autoantibodies in Juvenile Myositis Are Associated with Severe Disease Features and Mortality [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/myositis-associated-autoantibodies-in-juvenile-myositis-are-associated-with-severe-disease-features-and-mortality/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/myositis-associated-autoantibodies-in-juvenile-myositis-are-associated-with-severe-disease-features-and-mortality/