Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The prescription of Mycophenolate Mofetil (MMF) represents the primary treatment for interstitial lung disease (ILD) associated with systemic sclerosis (SSc) and is an option for cutaneous fibrosis. However, its use in patients with Limited Cutaneous SSc (lcSSc) is less common due to their lower fibrotic burden. In vitro observations and clinical data from kidney transplant patients suggest a protective effect of MMF on endothelial function. The objective of this study is to explore the impact of MMF therapy in preventing the escalation of vasoactive/vasodilator treatment as a surrogate for vascular complications of the disease.
Methods: Clinical data of patients with lcSSc enrolled in the Italian SPRING registry were retrospectively compared based on the prescription of MMF at any stage of their disease. Each patient treated with MMF for at least 3 months was then matched to another patient not treated with MMF and evaluated in the same quarter using a time-dependent propensity score. The score was based on age, gender, disease duration, presence of anti-centromere antibodies and anti-Scl-70+ antibody, ILD, pulmonary function tests, history of digital ulcers, and baseline treatment with iloprost, calcium channel blockers, endothelin receptor antagonists (ERA), and phosphodiesterase-5 inhibitors (PDE5i). Standardized mean differences (SMDs) after matching were reported as a measure of balance between the intervention and comparison group, with values ≤ 0.1 indicating good balance. The escalation of vasoactive/vasodilator treatment over up to 60 months was the considered outcome that was assessed as the addition of iloprost, ERA, or PDE5i due to uncontrolled or newly diagnosed peripheral vascular complications.
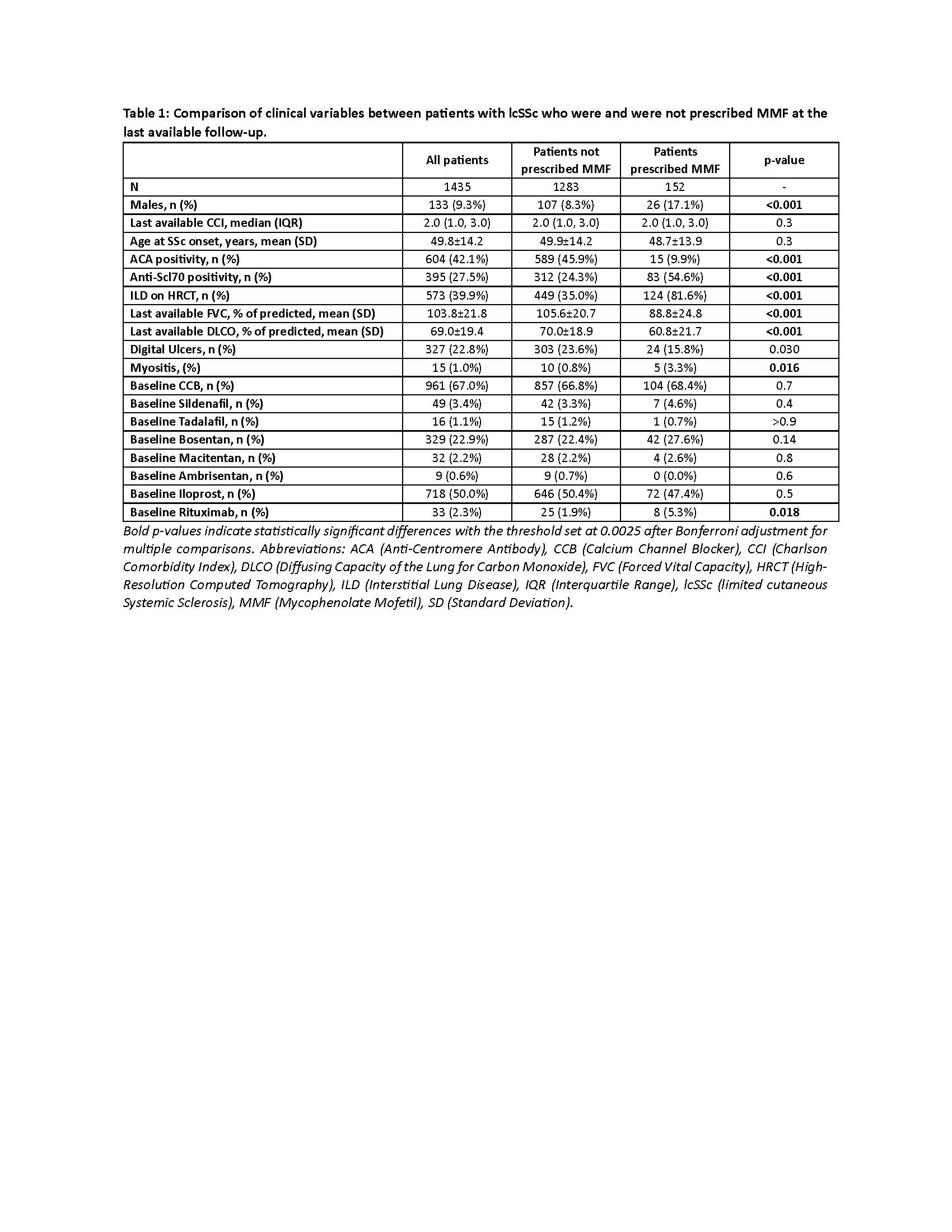
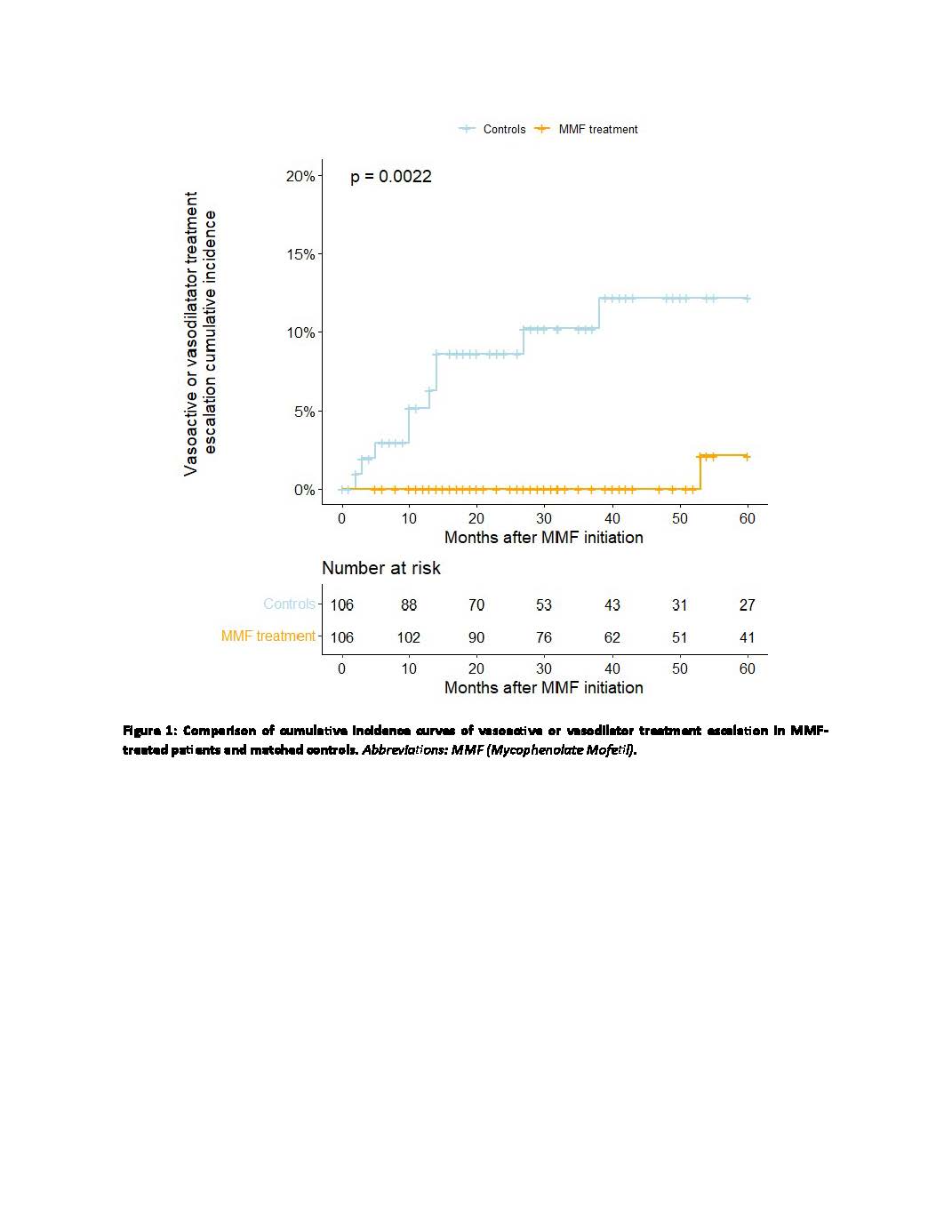
Results: Clinical characteristics of the evaluated 1,435 patients are reported in Table 1. The prescription of MMF was more common in male patients, those who were anti-Scl70 positive and anti-centromere negative, those with a history of interstitial lung disease or myositis, and those without a history of ulcers. Matching led to SMD< 0.1 for all the collected variables (data not shown). The overall incidence of vasoactive/vasodilator treatment escalation events over a median follow-up of 40.5 months (IQR 23.3-60.0) was 1.0 per 100 patient-years in the MMF-treated group and 7.3 per 100 patient-years in the control group, with a significant difference in treatment escalation-free survival between the two groups (Log-Rank test p = 0.002) (Figure 1).
Conclusion: In the national SPRING cohort, the decision to prescribe MMF is primarily driven by the presence of risk factors for progressive pulmonary manifestations. The association of MMF therapy with a reduced need for escalation of vasoactive or vasodilator treatment suggests a potential impact of this drug in preventing vascular complications in patients with lcSSc.
patients and matched controls. Abbreviations: MMF (Mycophenolate Mofetil).
To cite this abstract in AMA style:
De Lorenzis E, Natalello G, Cacciapaglia F, De Angelis R, Cipolletta E, Codullo V, De Luca G, Giuggioli D, Ingegnoli F, Riccieri V, D'Agostino M, Ferri C, Matucci Cerinic M, Bosello S. Mycophenolate Mofetil Treatment in Limited Cutaneous Systemic Sclerosis Reduces the Risk of Vascular Complication Leading to Treatment Escalation: Emulation of a Target Trial Using Time-dependent Propensity Score-matching [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/mycophenolate-mofetil-treatment-in-limited-cutaneous-systemic-sclerosis-reduces-the-risk-of-vascular-complication-leading-to-treatment-escalation-emulation-of-a-target-trial-using-time-dependent-prop/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/mycophenolate-mofetil-treatment-in-limited-cutaneous-systemic-sclerosis-reduces-the-risk-of-vascular-complication-leading-to-treatment-escalation-emulation-of-a-target-trial-using-time-dependent-prop/