Session Information
Date: Sunday, November 12, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes I: Biomarkers
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: SLE is one of the leading causes of death in young females suffering from autoimmune disorders. Nephritis, afflicts 60-70% of patients which contribute significantly to morbidity and mortality despite therapeutic advances. Disrupted cytokine networks and autoantibodies play an important role in disease pathogenesis. However, conflicting reports and non-reproducibility have hindered progress with regards to translational potential of cytokines. This study attempts to address the existing knowledge gap using a multiplex cytokine assay and machine learning algorithms.
Methods: 69 SLE patients fulfilling SLICC criteria were recruited. Baseline characteristics along with disease activity was recorded for all patients. Cytokines were measured by a commercially available multiplex cytokine kit (Millipore). Fluorescence values were logarithmically transformed before analysis. To visualize relationships between cytokines, Network graphs were constructed based on Spearman correlation values. For stratifying individuals based on cytokine profiles, we used Sparse Partial Least Squares Discriminant Analysis (sPLS-DA) followed by factor loadings plots to assess which cytokines contribute most to the observed differences between groups. Patterns of co-occurrence of autoantibodies within the SLE patients were identified by the K-Modes algorithm and the patients were visualized on a dimensionally reduced plot obtained by constructing a 2-dimensional representation by multiple correspondence analysis (MCA).
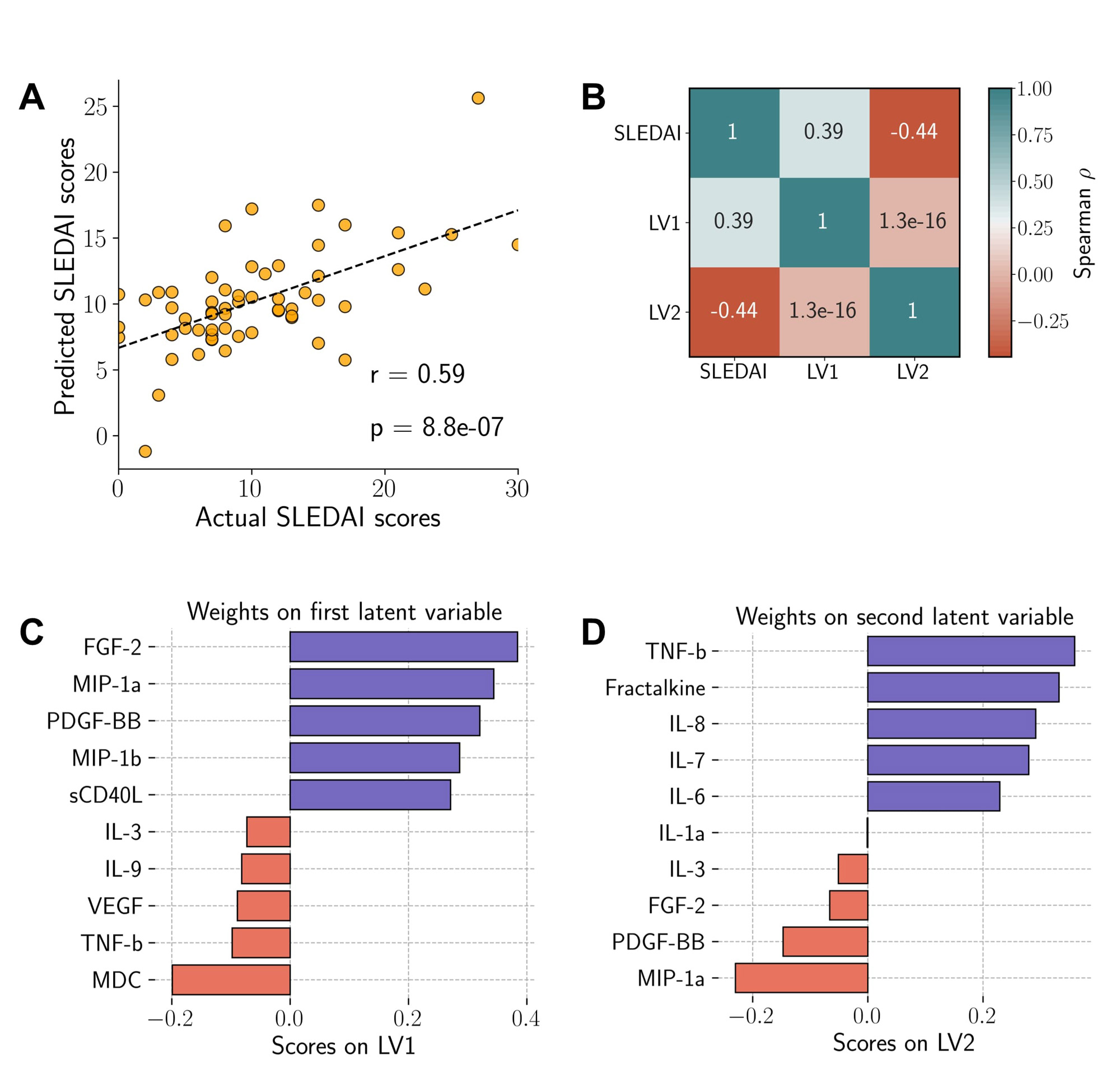
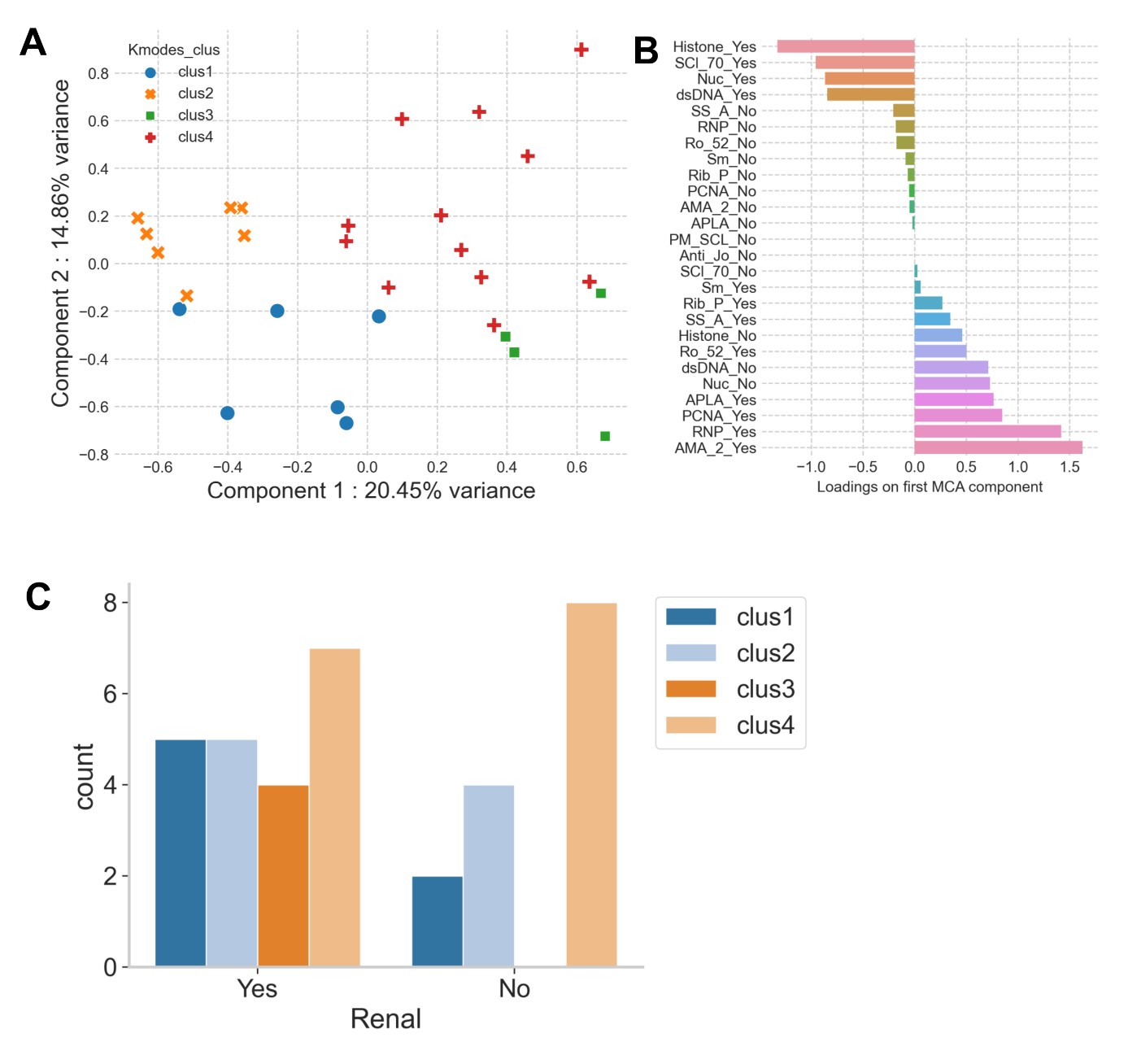
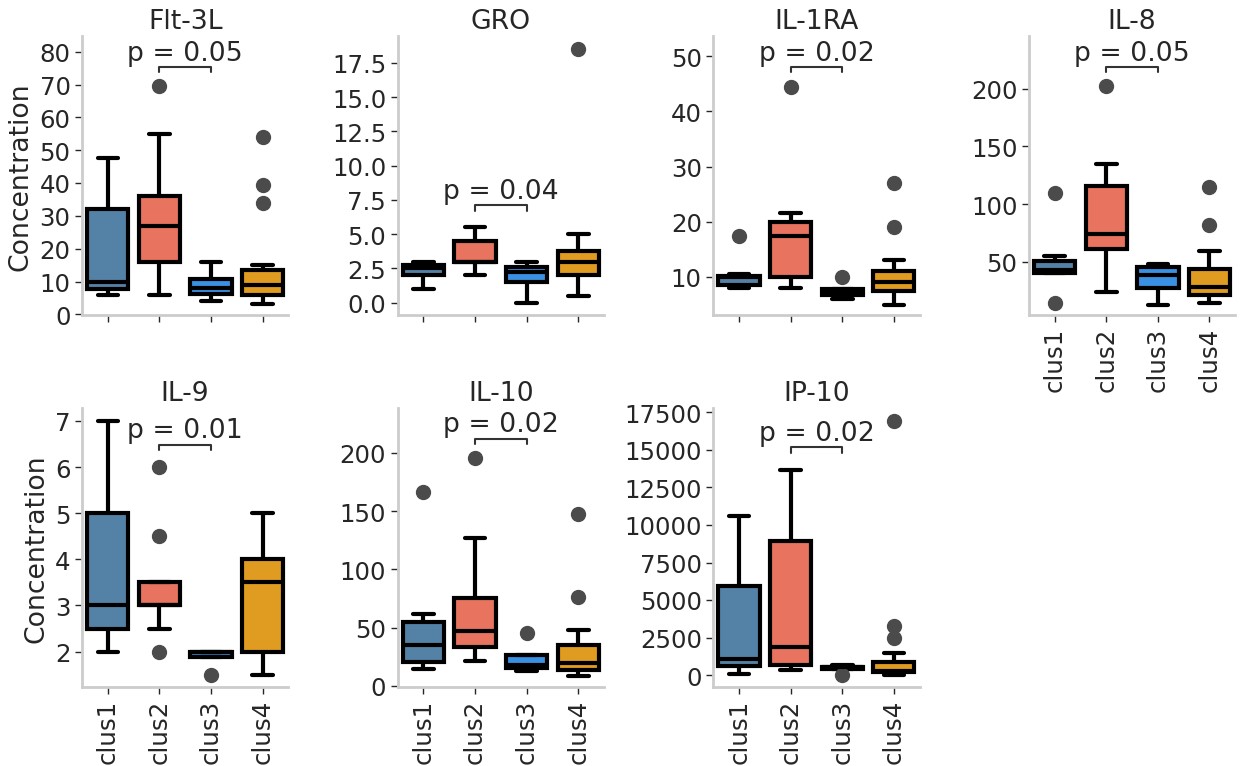
Results: We observed a positive association between actual disease activity scores (SLEDAI) and predicted scores from a partial least squares regression (PLSR) analysis of multivariate cytokine response data (Figure 1a). A study of the first two PLSR components revealed the SLEDAI scores to be positively associated with the first component (LV1) and negatively associated with the second component (LV2) (Figure 1b). Analysis of cytokine contributing towards the first two PLSR components revealed MIP-1α to have a strong positive influence towards component 1 and a negative influence on component 2, indicating a strong association with increased disease severity (Figure 1c and d). K-Modes clustering analysis identified 4 distinct clusters of patients with specific autoantibodies (Figure 2a and b), with clusters 2 and 3 being the most well-separated. Furthermore, we also observed striking differences in distributions of lupus nephritis between the clusters, with all patients in cluster 3 presenting with nephritis (Figure 2c). Finally, our results also demonstrate unique cytokine signatures associated with autoantibody profiles, with patients in cluster 3 showing significantly lower levels of cytokines responsible for modulating inflammation and maintaining cellular homeostasis (Figure 3), an observation that is in line with the clinical feature of nephritis.
Conclusion: Cytokine response can predict disease activity. Nephritis is associated with specific autoantibody profiles and cytokine signatures.
To cite this abstract in AMA style:
Pattanaik S, Mukherjee R, Tripathy R, Prusty B, Ravindran B, Das B. Multiplex Profiling and Machine Learning Reveal Distinct Signatures of Circulating Cytokines Associated with Autoantibody Profiles and Disease Severity in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/multiplex-profiling-and-machine-learning-reveal-distinct-signatures-of-circulating-cytokines-associated-with-autoantibody-profiles-and-disease-severity-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multiplex-profiling-and-machine-learning-reveal-distinct-signatures-of-circulating-cytokines-associated-with-autoantibody-profiles-and-disease-severity-in-systemic-lupus-erythematosus/