Session Information
Session Type: Abstract Session
Session Time: 12:15PM-12:30PM
Background/Purpose: Despite advances in rheumatoid arthritis (RA) treatment, 5–20% of patients experience persistent symptoms, particularly pain, and are classified as difficult-to-treat (D2T). Factors such as treatment resistance, comorbidities, and central pain sensitization may contribute; however, the exact mechanisms remain unclear. This study focused on investigating the underlying factors of the D2T condition and revealing the connections between pain and inflammation with a multimodal approach.
Methods: 31 D2T RA (determined by the 2020 EULAR definition), 18 non-D2T RA patients, and 32 healthy controls (HCs) were included. All participants underwent a clinical assessment, psychological analysis (including in-depth interviews, the Rorschach test, and questionnaires), two resting-state functional MRI (fMRI) scans with standardized heat pain stimulation in between, as well as peripheral blood transcriptomic analysis and plasma metabolomics.
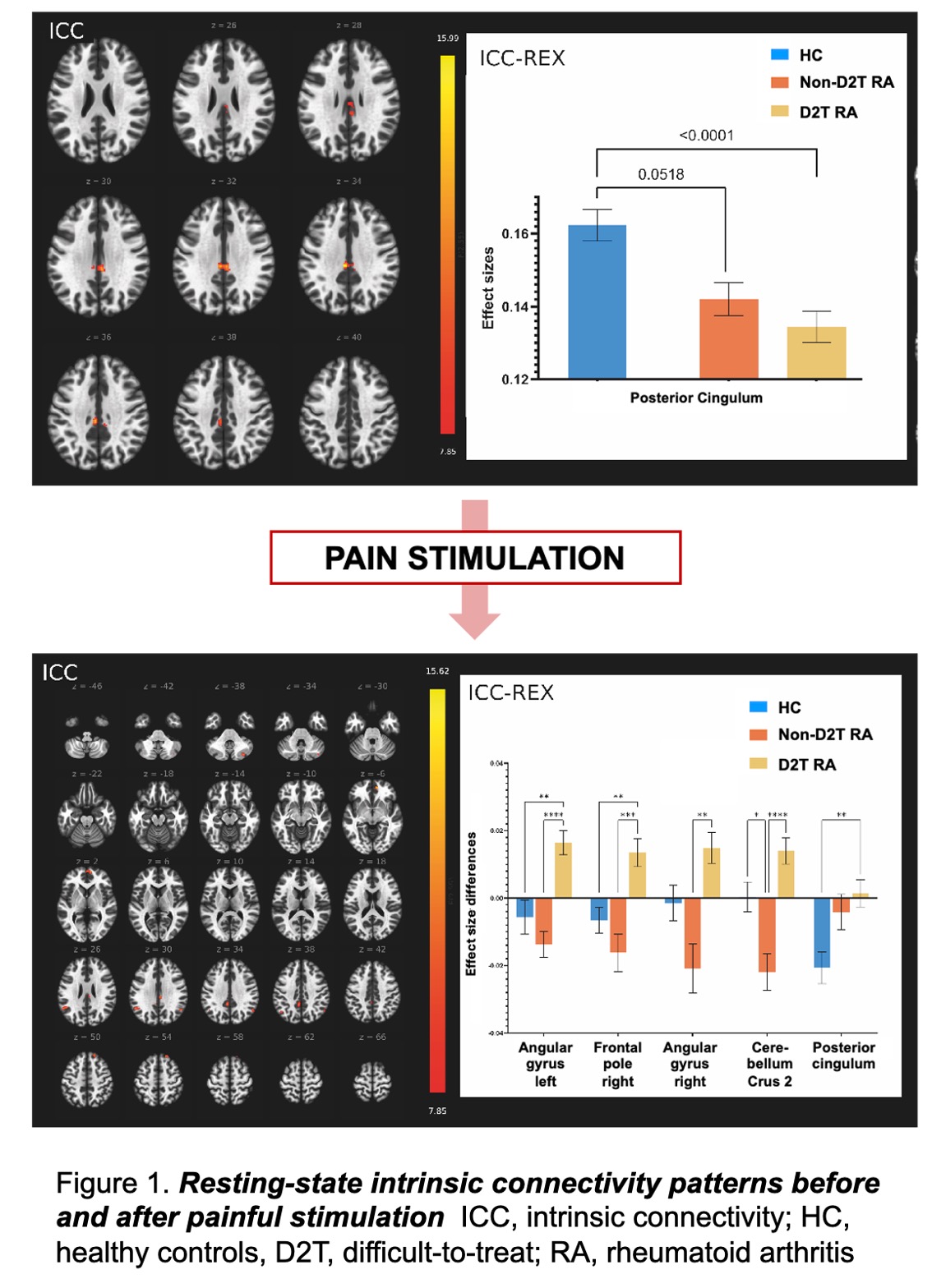
Results: Resting-state fMRI revealed significantly reduced intrinsic connectivity of the posterior cingulate cortex (PCC) in RA patients, especially in D2T RA. Following acute painful stimulation, intrinsic connectivity of the PCC increased in D2T RA, in contrast to the decreased connectivity observed in non-D2T RA patients and healthy controls (HCs). (Figure 1.) Seed-based analysis of the postcentral gyrus (PCG) revealed a reduction in connectivity strength across many connections within the somatosensory area, which were significantly altered after pain stimulation in all RA patients compared to HCs. Fractional amplitude of low-frequency fluctuations (fALFF) analysis revealed significantly reduced activity in several brain regions (e.g., lateral occipital cortex, middle frontal gyrus, medial prefrontal cortex, and frontal pole) in RA, with D2T patients showing the most significant reductions. Compared to non-D2T RA, D2T patients also exhibited lower fALFF in additional brain regions, indicating more complex alterations in pain and somatosensory processing, behavior, and cognitive functions, which aligns with our psychological results showing increased relational and decision-making inhibition, reduced motivation, and impaired mental control in RA. (Figure 2.) Transcriptomic analysis showed 70 downregulated and 17 upregulated differentially expressed (DE) mRNAs in D2T RA compared to the non-D2T RA group (e.g., neuregulin-1 (NRG1) or S100B). Functional analysis demonstrated a direct link of DE mRNAs to neuronal differentiation processes, neuroinflammation, or neuroplasticity. (Figure 3.) Metabolomic analysis revealed significant differences in amino acid and sphingolipid metabolism.
Conclusion: Teveal a novel neuroimmune signature of D2T RA, characterized by altered brain connectivity patterns, psychological disturbances, and distinct transcriptomic and metabolomic profiles. These results may lead to new opportunities for patient stratification, biomarker development, and personalized interventions targeting central mechanisms.Funding: HUN-REN–PTE Chronic Pain Research Group (14017), OTKA K138046 and K131479, TKP2021-EGA-29, National Brain Research Program, Richter Gedeon PhD Scholarship for LGT.
To cite this abstract in AMA style:
Gunkl-Tóth L, Orsi G, Császár-Nagy N, Duzsik L, Mátay G, Kumánovics G, Sütő G, Csókási K, Takács S, Vidnyánszky Z, Kun J, Takács-Lovász K, Karvaly G, Farkas R, Pintér A, Királyhidi P, Nagy G, Helyes Z. Multimodal Analysis Revealed Altered Brain Connectivity Patterns and Neuroinflammatory Processes in the Background of Difficult-To-Treat Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/multimodal-analysis-revealed-altered-brain-connectivity-patterns-and-neuroinflammatory-processes-in-the-background-of-difficult-to-treat-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multimodal-analysis-revealed-altered-brain-connectivity-patterns-and-neuroinflammatory-processes-in-the-background-of-difficult-to-treat-rheumatoid-arthritis/

.jpg)
.jpg)