Session Information
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: First-degree relatives of RA patients (FDR-RA) have an increased prevalence for rheumatoid arthritis (RA) [1] and joint symptoms [2]. Identification of individuals with imminent RA is key to achieve prevention in pre-clinical stages.
We developed a two-step approach combining established biomarkers including different isotypes, genetics and first symptoms (step 1) followed by multiple and high positivity for serologic marker (step 2) to identify FDR-RAs with a high risk to develop RA. Using this approach to identify patients before onset of disease could improve patient management in the future.
Methods: 1227 FDR-RAs from the Swiss multicenter cohort study SCREEN-RA were included in this study [3]. The established serologic biomarkers anti-CCP IgG/A, RF IgM/A (CE-IVD) and the prototype assay anti-RA33 IgM/A/G were measured. The NGS technology AmpliSeqTM on the Ion GeneStudioTM instruments (Thermo Fisher Scientific, USA) was used covering 320 variants specific for RA in a targeted sequencing run. Symptoms of the patients were included using information from the Clinical Suspected Arthralgia (CSA) questionnaire. An algorithm based on Naïve Bayes was developed to combine serology, genetics and symptoms and in addition multiple and high positivity to define risk scores for each criterion for the included individuals. Individuals which had a high risk score for ≥2 criteria were flagged, as well as individuals with high or multiple positivity for the included biomarkers.
Results: For each individual the risk score for the three criteria (serology, genetics and symptoms) was calculated. 86 individuals (7%) were identified at high risk for developing RA including 51 individuals being positive for ≥ 2 criteria (step 1; Fig 1) and additional 35 individuals showed multiple positivity and/or high positivity for serologic markers (Fig 2). Individuals which were flagged during step 1 of the approach were not included in the numbers for step 2. Among 13 individuals diagnosed with incident clinical RA by rheumatologists, 9 of them (70%) were correctly identified by our approach.
Conclusion: The newly created algorithm including various risk factors could support the identification of individuals at risk to develop the disease in the near future and could allow a tight control. The highlighted individuals (n=86) will be followed up to identify imminent RA.
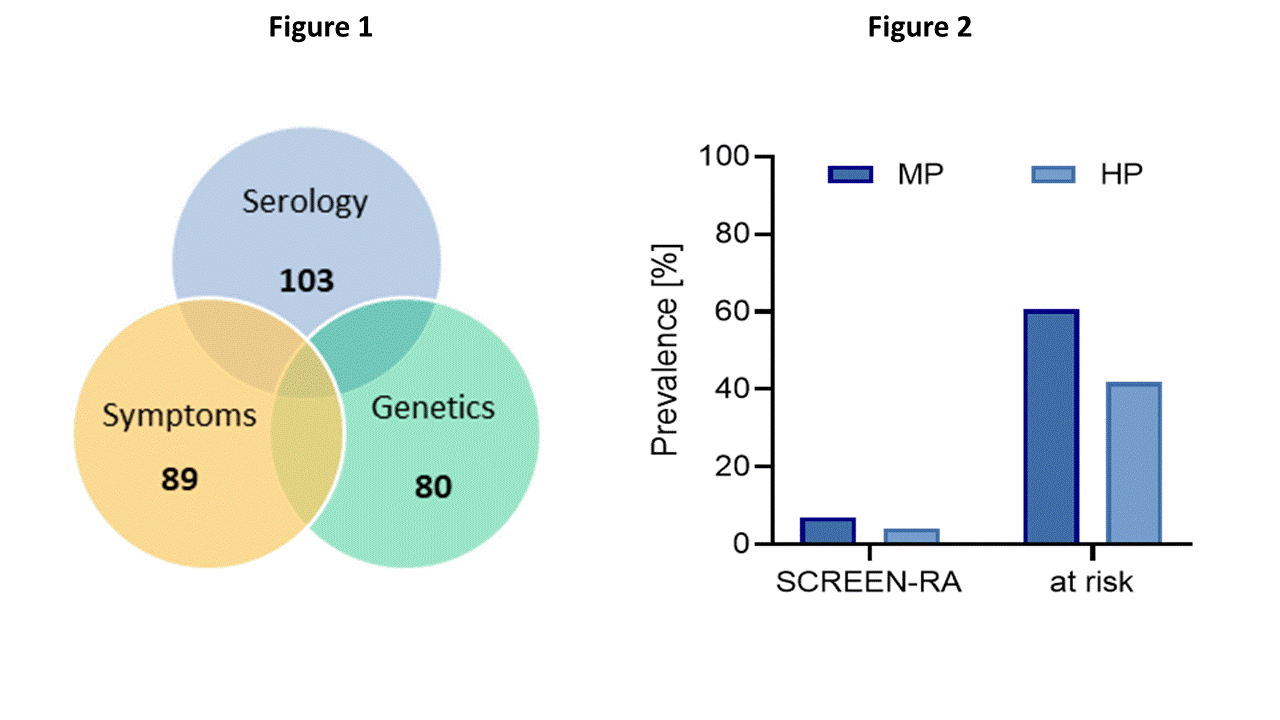 Fig 1: Venn diagram showing the identified individuals for each criterion and the individuals with ≥2 criteria positive. During step 1 of the approach, 51 individuals (4%) were highlighted as at risk. Fig 2: Additional 35 individuals (3%) were flagged during step 2 of the approach showing high or multiple positivity for the measured biomarkers
Fig 1: Venn diagram showing the identified individuals for each criterion and the individuals with ≥2 criteria positive. During step 1 of the approach, 51 individuals (4%) were highlighted as at risk. Fig 2: Additional 35 individuals (3%) were flagged during step 2 of the approach showing high or multiple positivity for the measured biomarkers
To cite this abstract in AMA style:
Lamacchia C, Grundhuber M, Gehring I, Roux Lombard P, Nissen M, Rubbert Roth A, Mueller R, Walker U, Moeller B, Kyburz D, Ciurea A, Swiniarski S, Finckh A. Multi-Variate Approach Including Serology and Genetics for an Improved Identification of Patients at Risk of Developing Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/multi-variate-approach-including-serology-and-genetics-for-an-improved-identification-of-patients-at-risk-of-developing-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multi-variate-approach-including-serology-and-genetics-for-an-improved-identification-of-patients-at-risk-of-developing-rheumatoid-arthritis/
