Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Macrophage activation syndrome (MAS) is a complication of systemic juvenile idiopathic arthritis (sJIA) characterized by cytokine storm and overt immune cell activation. We aim to further understand the immunologic landscape of sJIA and MAS.
Methods: We profiled peripheral blood mononuclear cells (PBMCs) from patients with active sJIA with or without MAS at the time of sampling and age-matched controls using bulk RNA sequencing (RNA-seq), single-cell RNA-seq, and mass cytometry. All patients with sJIA met ILAR diagnostic criteria and MAS was defined according to the 2016 Classification criteria for MAS complicating sJIA. Findings from RNA-seq and mass cytometry were validated using additional samples from patients with sJIA as well as patients with other inflammatory diseases by flow cytometry and in vitro T cell stimulation studies.
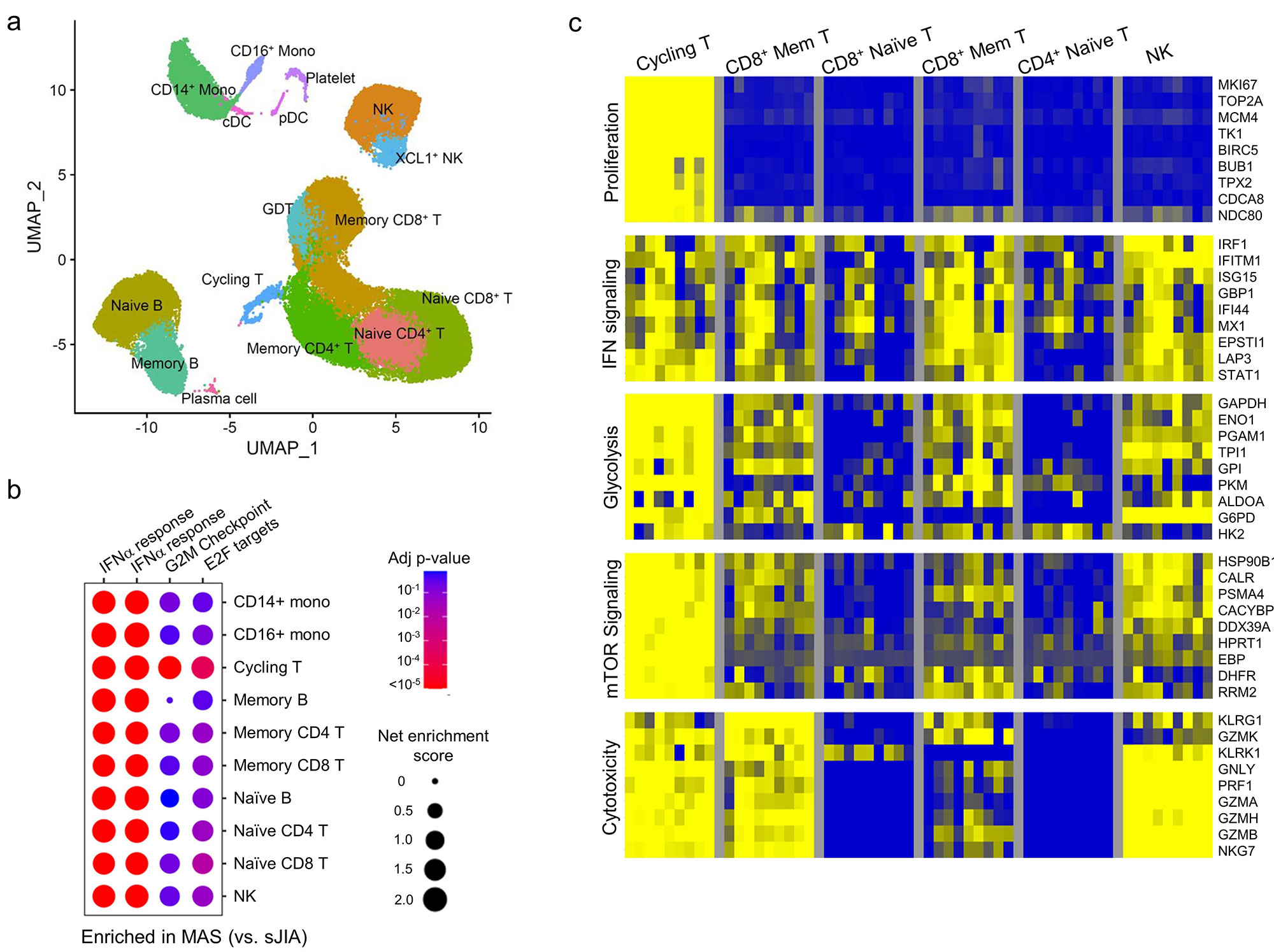
Results: Gene set enrichment analysis of bulk RNA-seq of PBMCs from patients with MAS (n = 9) revealed strong expression of genes associated with type I interferon signaling and T cell proliferation in addition to the expected type II IFN (IFN-g) signature based on gene set enrichment analysis. These features were rarely seen in age-matched healthy controls (n = 18) or patients with sJIA without MAS (n = 15). Single-cell RNA-seq confirmed the presence of type I and type II IFN signatures on all major PBMC subsets in patients with MAS (n = 8) while the T cell proliferation signature was localized to a population of Ki67+ cycling T cells with enhanced expression of IFN-g and genes involved in glycolysis, mTOR signaling and cytotoxicity (Figure 1).
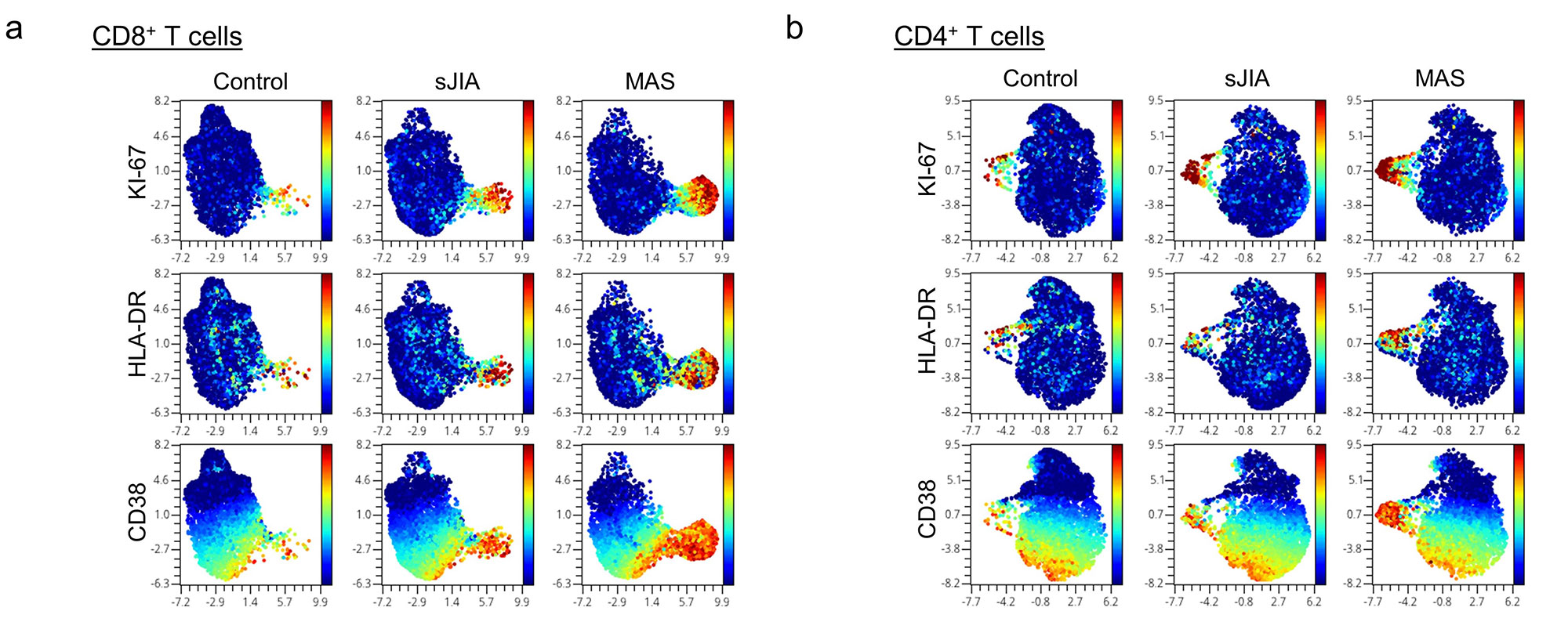
Mass cytometry revealed that the cycling T cell population comprised of predominantly CD8+CD38+HLA-DR+ T cells and fewer CD4+CD38+HLA-DR+ T cells (Figure 2). We found that an expansion of CD38+HLA-DR+ T cells beyond 9% of CD8+ T cells or 4% of CD4+ T cells distinguished MAS from active sJIA, while a milder increase was generally seen in patients with sJIA without MAS compared to healthy controls (Figure 3). The degree of CD8+CD38+HLA-DR+ T cell expansion was also significantly greater in MAS compared to multi-system inflammatory syndrome in children (MIS-C) associated with COVID-19 (n = 17), acute viral infections (n = 15), Kawasaki disease (n = 7) and systemic lupus erythematosus (n = 9). In addition, stimulation of isolated T cells from healthy donors with IFN-I, but not IFN-II, augmented the effects of IL-12, IL-15 and IL-18 to generate CD8+CD38+HLA-DR+ T cells in vitro, while Janus kinase inhibition by ruxolitinib mitigated the impact of IFN-I treatment.
Conclusion: Our multi-omic analysis further details the expansion of the recently described CD8+CD38+HLADR+ T cells in MAS associated with sJIA. Our findings demonstrate a synergistic role of IFN-I in the generation of these cycling T cells and provide a mechanistic link between viral infections and MAS that may be targetable by JAK inhibition.
To cite this abstract in AMA style:
Brodeur K, Chen L, huang z, Du Y, Wobma H, Taylor M, Chang J, Day-Lewis M, Dedeoglu F, Halyabar O, Lo M, Newburger J, Son M, Sundel R, Nigrovic P, henderson l, Lee P. Multi-omic Analysis of Macrophage Activation Syndrome Associated with sJIA Reveals a Potential Role of Type I Interferons in the Expansion of Cycling T Cells [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/multi-omic-analysis-of-macrophage-activation-syndrome-associated-with-sjia-reveals-a-potential-role-of-type-i-interferons-in-the-expansion-of-cycling-t-cells/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/multi-omic-analysis-of-macrophage-activation-syndrome-associated-with-sjia-reveals-a-potential-role-of-type-i-interferons-in-the-expansion-of-cycling-t-cells/